When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.


When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

There are several steps that you can take to ensure that you get the greatest benefit from consultants.

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

Now is a good time for companies to know their suppliers well.

The supply base for preclinical and clinical development services continues to expand in China.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

How an electronics engineer led the first Indian company to carry out indigenous development of a recombinant vaccine.

The cause of the heparin crisis is still unknown. We do know, however, that the FDA is severely underfunded.
Indian biogenerics could form a major piece of the global biotherapeutics market in the future.

A CMO faces significant risk of lost revenues and profits if the product fails in clinical trials or doesn't meet sales projections.

How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.

China's pharma industry represents 5% of the world total. By 2010, China is expected to become the world's fifth largest pharmaceutical market.
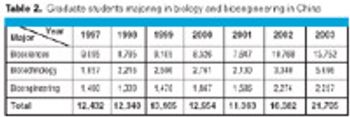
Biotechnology is definitely a hot topic in China-the country's administrators recently identified it as a "cornerstone of China's national economy by 2020." But most realize that getting there will require a better trained, specialized workforce than currently exists. The Chinese government has been pumping money into life sciences education as part of its plan to achieve a global biotechnological presence over the next 15 years.

The approval of a new seasonal influenza vaccine has further diversified the supply to the US market.

Cobra Biomanufacturing is an international full-service manufacturer of biopharmaceuticals, dedicated to designing robust processes that deliver biopharmaceutical products to its life sciences customers for preclinical through Phase 3 studies.

Discovery Laboratories (Warrington, PA) could see the end of its struggle to launch its Surfaxin (lucinactant) drug on the US market soon, as the manufacturing issues it has faced have been resolved.

There wasn't much of a contract services industry when BioPharm International began publishing 20 years ago. Today's big names in biomanufacturing, including Lonza, Boehringer-Ingelheim, and Avecia, had not yet entered the business.
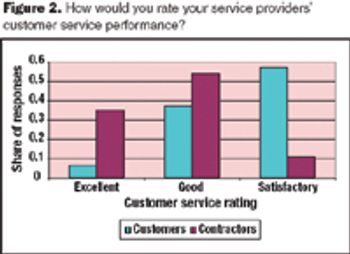
Meeting service levels is a major challenge for pharmaceutical services providers because the requirements of their client base vary widely.

From the earliest days of the biotechnology industry, companies have grappled with the complexities of making innovative biopharmaceuticals on a large scale. Success in manufacturing begins with process science, since biotech production requires perfection in maintaining living organisms in a sterile environment under controlled physiological conditions. But unless companies can solve the challenge of planning for and managing manufacturing capacity, they will not be able to achieve the full potential of promising biotech products.

The center of gravity for the pharmaceutical market is shifting to the Asia-Pacific region from the US and Europe, according to an analysis published by PricewaterhouseCoopers (New York, NY, www.pwc.com).

The US Food and Drug Administration (FDA, Rockville, MD, www.fda.gov) issued a revised draft guidance on July 20 to help ensure that the safety, purity, and potency of biologics products is not compromised as a result of innovative, flexible manufacturing arrangements.
The corruption of China's food and drug sectors is not limited to one man. Likewise, real reform will require the efforts of many.
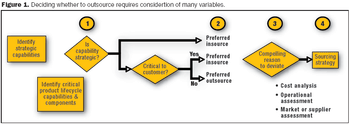
Companies need to avoid operating in a manner that is inconsistent with the priorities established in the strategic sourcing decision.
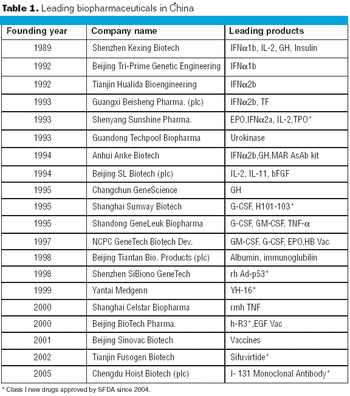
In 2006, China exported a total of $890 million in biologics to other countries-a 30.61% increase, compared with the previous year.

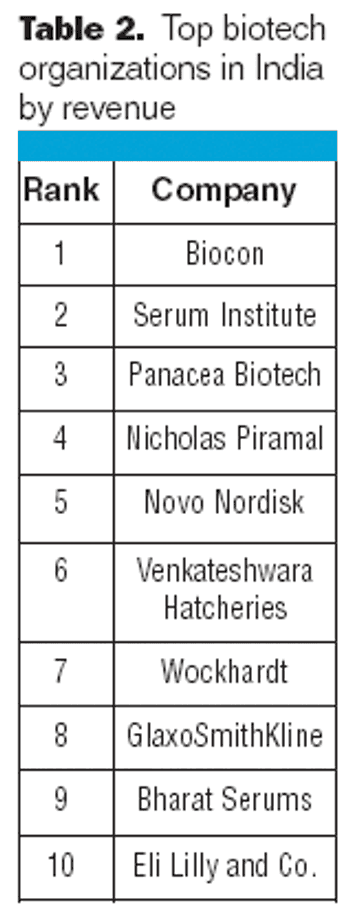
Indian biotech revenues grew almost 31% to more than $2.08 billion in 2006–2007, according to a recent report.