
An expanded pharma services supply chain facility in Germany boosts Thermo Fisher’s Pharma Services footprint in Europe.

An expanded pharma services supply chain facility in Germany boosts Thermo Fisher’s Pharma Services footprint in Europe.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

The new 73,000-square-foot facility is one of several expansions to support the company’s biologics testing capabilities.

Contract testing organizations can provide bio/pharma companies with a cost-effective way to adapt to new technologies and regulations.

A review of the latest investments in facilities, equipment, and acquisitions by biopharma contract service providers.
A different perspective on controlling fixed costs of biomanufacturing, based on know-how from other industries, provides a competitive edge, says the CEO of Samsung BioLogics.
Successful outsourcing relationships for early phase analytics in drug development are driven by partnership.

Research suggests that working with a single contract partner can reduce development time and improve economics.

Grand River Aseptic Manufacturing announces first planned investment in capacity expansion.

Catalent completes $4.6 million expansion at Singapore clinical supply facility and marks 20 years in the region.
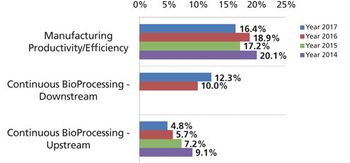
Development and adoption of new technologies create challenges that may take years to resolve.

The contract development and manufacturing organization has entered it first manufacturing contract worth $148 million for its recently completed Plant 3 facility.

The company aims to add the additional analytical services in the European Union throughout 2018.

Avid Bioservices will provide commercial manufacture of an enzyme replacement therapy by Roivant Sciences' Enzyvant subsidiary.

Unlike current approaches where bioconjugation is typically done following the manufacture of the monoclonal antibody and the cytotoxic drug, the new method begins with the antibody supernatants and eliminates the need for extensive chromatographic purification.

A $800-million acquisition of MPI Research expands Charles River's offerings for early-stage contract research.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.

Biopharm industry veteran Ralf Otto named to lead development and manufacturing at Rentschler Biopharma.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.

Frustrated with high costs and drug shortages, hospitals adopt a DIY approach.

Greater clarity on the application of existing regulations will accelerate development of cell and gene therapies.

The companies will be integrated to form the newly-branded AGC Biologics, which will specialize in customized contract development and manufacturing organization services for the scale-up and cGMP manufacture of protein-based therapeutics.

The contract development and manufacturing organization has invested £30-million (US$42 million) at its Dundalk, Ireland site to offer expanded clinical trial services.

The company relocated its headquarters to Riverview, Florida.

KBI Biopharma's acquisition of Elion Labs expands KBI's biophysical and analytical characterization capabilities.