
The second phase of a $5.5-million expansion adds controlled-substance and controlled-temperature storage to Catalent clinical trials facility.

The second phase of a $5.5-million expansion adds controlled-substance and controlled-temperature storage to Catalent clinical trials facility.

Valerius Biopharma will use Catalent’s GPEx technology to produce cell lines for biosimilar drugs.

The companies have extended a contract agreement for the commercial manufacture of the active pharmaceutical ingredient for vonapanitase, an investigational drug intended to improve hemodialysis vascular access outcomes.

Lonza announces addition of mid-scale biologics manufacturing capacity and cell-therapy suites at Portsmouth, NH site.

The company has begun expansion efforts for its process-development capabilities and laboratory infrastructure.

More published data and initial regulatory approvals are needed to drive adoption of continuous bio-manufacturing.

CDMOs can claim credit for the robust growth of emerging bio/pharma financings.

The company unveiled three new products to support single-use biomanufacturing and won an award for best technological innovation at INTERPHEX 2018.

The company is launching a new pre-fabricated, ready-to-run manufacturing facility that is expected to significantly decrease the production timeline for viral vector-based therapeutics.

A new report gives an overview of the work of the International API Inspection Program.

A $5.5-million expansion at its Philadelphia, PA clinical supplies facility gives Catalent additional packaging and storage capacity.

FDA sent a warning letter to Tris Pharma Inc. after investigators found the company had failed to properly investigate batch failures and establish quality control procedures.
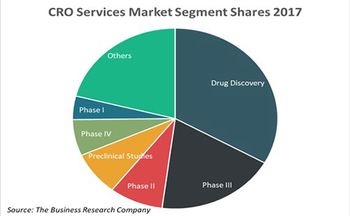
A new study by the Business Research Company reveals prominent contract research organization outsourcing trends.

The new 300,000-square-foot facility is considered the largest dedicated cell and gene therapy manufacturing facility with fully integrated services.

The contract manufacturing organization’s facility in Boulder, CO, has passed general inspection from FDA.

On Tuesday, April 24, 2018, Evan Boswell, senior principal scientist at Pfizer CentreOne Contract Manufacturing, Pfizer CentreOne will give a presentation on scaling up the manufacturing process of active pharmaceutical ingredients at CPhI North America in Philadelphia, PA.

Anthony Qu, PhD, vice president of Scientific Affairs at Halo Pharma, will give a presentation on fixed-dose combination products, drug products containing multiple active ingredients, as an effective approach for simplified dosing at CPhI North America on Wednesday, April 25, 2018 in Philadelphia, PA.

The contract development and manufacturing organization announced the addition of a new building complex that will house its headquarters in Bothell, WA.

Fujifilm acquires cell culture media companies Irvine Scientific Sales Company and IS Japan.

The active pharmaceutical ingredient and excipient provider has expanded its parenteral ingredient manufacturing capacity and lab services.

The contract service provider will invest in a laboratory expansion at its site located in Tredegar, Wales, UK.

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

Early adopters are benefiting from lower costs and increased productivity.

Certificates of analysis can be used to monitor the reliability of products and their suppliers, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.
Developments and investments in single-use systems advance upstream biomanufacturing.