
Getting the science right helps biopharma startups overcome development and commercialization challenges.

Getting the science right helps biopharma startups overcome development and commercialization challenges.

A look at the skill sets and training needed to tackle the increasing levels of automation in bioprocessing facilities.

The company has launched a comprehensive portfolio of custom cell biology solutions and a new cell-line engineering technology.

Belgium-based CDMO MaSTherCell will expand its European manufacturing capacity for cell and gene therapy products by setting up a new facility in Belgium.

A $1.7-billion acquisition CDMO Brammer Bio establishes Thermo Fisher Scientific in viral vector manufacturing.

The companies aim to assess automated CAR-T cell therapy manufacturing at the point-of-care and develop technologies to facilitate patient access to immunotherapies.

The Pharma Services business of Thermo Fisher Scientific will invest $150 Million at three facilities.

The acquisition of the site in Copenhagen, Denmark, will significantly expand Fujifilm’s capacity and capabilities.

Technology vendors are strengthening their positions in China as the nation emphasizes self-sufficiency in research, development, and manufacturing.
Single-use systems can be a cost savings for CMOs, and these savings can be passed on to clients and, ultimately, to patients.
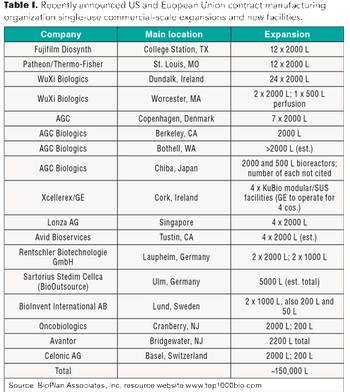
A look at recent investments by contract manufacturers to increase single-use bioreactor capacity.

Catalent adds position of president and chief operating officer to lead sales and quality efforts.

The US District Court for the Western District of Pennsylvania entered into a consent decree of permanent injunction against Ranier’s Rx Laboratory Inc., a compounding facility.

PCI Pharma Services expanded capacity of its commercial packaging site in Illinois.

Radha Iyer, vice-president and head of Quality and Scientific Affairs, Global Developed Markets, Dr. Reddy’s Laboratories, discusses new initiatives at the company.

Company launches new services dedicated to emerging biotech and biopharma companies.

Suppliers address the complexity of supplying disposable components for single-use systems to the global biopharmaceutical manufacturing industry.

Single-use components for biopharmaceutical manufacturing have a lower environmental impact than reusable components, but disposal is still a consideration.

SGS announces expansion of cell bank and bulk harvest testing services.

CDMO leader Marc Funk will succeed Ridinger as CEO of Lonza.

The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.

Fujifilm increases capacity of its Bio-CDMO business with an expansion of production in North Carolina.

The Massachusetts site, formerly associated with an affiliate of Shire, is Rentschler Biopharma’s first facility in the US.
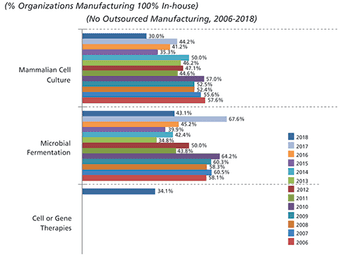
Outsourcing of manufacturing activities is expected to increase in 2019.

The company is investing approximately $14 million to expand biologics packaging capabilities and capacity at its biologics manufacturing facility in Bloomington, IN.