
The company successfully produced cell-based influenza vaccines at a commercial scale in its facility in NC.

The company successfully produced cell-based influenza vaccines at a commercial scale in its facility in NC.

The annual meeting of the Honeywell Users Group for the Americas will discuss industrial automation.

The company has voluntarily recalled Clindamycin Injection USP ADD-Vantage Vials to the hospital/retail level because of a lack of sterility assurance.

Sartorius Stedim Biotech combined the company’s ambr 15 bioreactor system with the Nova BioProfile FLEX2 cell culture analyzer for laboratory experiments.

FDA sent a warning letter to drug compounder DCA, Inc. dba Beacon Prescriptions for failing to ensure sanitary conditions.

The new UPS facility in Columbia will serve the growing pharmaceutical, biopharma, and medical device industry in Latin America.
The success of a truly integrated continuous processing platform relies on the collaborative efforts of upstream and downstream specialists.
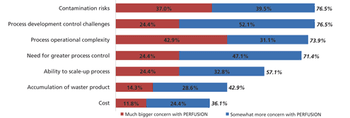
Although widespread adoption of continuous bioprocessing has been slow, some processes have been an exception.
This article summarizes the approaches, challenges, and future perspectives for the characterization of N-glycans in biopharmaceutical products.

Approval of breakthrough therapies requires expedited quality assessment.

As more companies decouple drug substance from finished drug manufacturing operations, an integrated approach can ensure safe, reliable logistics for frozen storage and shipping.
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

BioVectra will open its new microbial fermentation and complex chemistry site, including the capability to handle high-potency APIs, by the end of 2017.

A recent survey suggests that 36% of pharmaceutical companies and contract development and manufacturing companies have not started working on serialization, and that those who are working on it are focusing on basic compliance rather than potential long-term business benefits.

The authors examine the selection process of the contract manufacturing–pharmaceutical relationship and the communication challenges that pharmaceutical firms face prior to and during the CMO selection process.
Andrea Zobel, senior director of product management, clinical trial supply, and logistics at PAREXEL International, examines key issues and questions that sponsors and contract partners must address.

Good project management, budgeting, planning, and clear documentation are the only ways to prevent overruns and project failure.

BioPharm International speaks with a CMO to find out how it is coping with capacity challenges, regulatory authority scrutiny, supply chain reliability, and new requests from clients to incorporate continuous manufacturing into processing lines.

FDA sent a warning letter to Pharmaceutic Labs, LLC for deficiencies in producing sterile drugs.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.

Can bioprocessing runs be consistently replicated in an inherently variable production environment?

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.

Avid, a wholly owned subsidiary of Peregrine Pharmaceuticals, will upgrade its Myford, California clinical and commercial manufacturing facility with multiple Mobius 2000-L single-use bioreactors from MilliporeSigma, the companies announced on May 1, 2017.

Recent legislation and PDUFA initiatives aim to streamline oversight and testing requirements.