
Johnson & Johnson Supply Chain (JJSC) and the distributor AmerisourceBergen launched a four-week pilot program to test GS1’s EPCIS standards and to see how effectively data could be transferred between the two partners.

Johnson & Johnson Supply Chain (JJSC) and the distributor AmerisourceBergen launched a four-week pilot program to test GS1’s EPCIS standards and to see how effectively data could be transferred between the two partners.

The author discusses the results from TraceLink and Actionable Research's Global Drug Supply, Safety and Traceability Report.

Avella issued a nationwide recall of sterile products produced at the Advanced Pharma Houston location due to inaccurate labeling.

Sanofi and Lonza formed a joint venture to build and operate a large-scale mammalian cell culture facility for monoclonal antibody production in Visp, Switzerland.

The company is voluntarily recalling one lot of Edex due to a lack of container closure integrity.

The companies have developed a Level 4 traceability solution to manage pharmaceutical regulatory requirements.

FDA granted inotuzumab ozogamicin priority review and accepted its BLA for filing.

CellGenix will add R&D, production, and warehouse space in Freiburg, Germany for GMP-grade raw materials for cell therapy, gene-therapy, and tissue-engineered products.

In a press release, Momenta announced on Feb. 16, 2017 that Momenta/Sandoz’s fill/finish contract manufacturer, Pfizer, received a warning letter for the manufacture of the 40 mg of Glatopa (glatiramer acetate injection), Momenta’s generic version of the drug Copaxone. Pfizer said in the statement that the warning letter does not affect the production or shipment of the 20 mg version of the drug, which is already approved by FDA.

Under the agreement, Abzena will manufacture magacizumab, an antibody created using the ‘Abzena inside’ Composite Human Antibody technology.
Process controls get some upgrades to better reflect real-time conditions.

FDA sent a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found deviations in the facility’s aseptic processes.

The company has voluntarily recalled all lots of of human chorionic gonadotropin because of a lack of sterility assurance.

The agency sent a warning letter to Resonance Laboratories Pvt. Ltd. after an inspection found possible contamination problems.
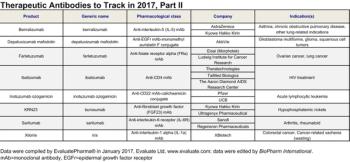
Evaluate and BioPharm International highlight the antibody-based therapeutics that may have 2017 launch dates in the United States.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.

This three-year partnership will explore and identify new tools and methods to modify and optimize the Chinese hamster ovary (CHO) cell line performance.

On a recent call, Catalent revealed that it has reached more than 90% of its current capacity and discussed how tax policy changes could affect the outsourcing industry.

According to results from the FOURIER trial, Repatha significantly reduced the risk of cardiovascular events and death in patients with atherosclerotic cardiovascular disease.

Patients with relapsing-remitting multiple sclerosis (MS) who are treated with currently available disease-modifying drugs (DMARDs) usually experience disease reactivation within the first five years of treatment follow-up. Many of the available treatments for MS only confer complete control of disease activity in a small percentage of patients.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.

Pump systems must be designed to meet the needs of specific processes, including preventing cross-contamination and damage due to shear forces.

To prevent failure during lengthy use, tube life should be monitored and a preventive maintenance program enacted.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.