
The companies have entered into a strategic collaboration to establish a new cell therapy and regenerative medicine manufacturing platform, which includes a new manufacturing facility in Belgium.

The companies have entered into a strategic collaboration to establish a new cell therapy and regenerative medicine manufacturing platform, which includes a new manufacturing facility in Belgium.

The collaboration will focus on developing a novel and differentiated challenge model for respiratory syncytial virus.

Europe updates the guideline on excipients information in labeling and packaging.

Johnson & Johnson’s Janssen Sciences Ireland UC will invest more than EUR 300 million (US$355 million) to expand manufacturing capacity for biologic medicines at its Ireland facility.

Hitachi Chemical Advanced Therapeutics Solutions will expand its PCT service platform for cell therapy by adding GMP manufacturing and cleanroom capacity in Allendale, NJ.

Under an agreement, ProBioGen will develop a stable cell-line for and manufacture an anti-cancer antibody for Chiome using its proprietary cancer cell-killing technology.

Groninger will showcase its FlexPro 50 line for small batch production at CPhI Worldwide 2017, and present on up- and downstream processes with freeze-dryer developer and manufacturer Martin Christ.

The Danish biotechnology company has been awarded a sole-source BARDA contract valued at more than $539 million for a freeze-dried smallpox vaccine.

Under Project BioShield, the agency could provide more than $170 million in funding to purchase and support late-stage development of Ebola vaccines and therapeutic drugs.

Genentech, AmerisourceBergen, and McKesson are providing pharma user requirements for a new project that will evaluate blockchain‘s potential in meeting requirements of the Drug Supply Chain Security Act, and preventing pharmaceutical counterfeiting.

GE Healthcare opened a 3D printing lab in Sweden that will speed the launch of products for bio/pharma manufacturing, such as a custom chromatography column.

Virgin Atlantic Cargo and Delta Cargo have opened a new Pharma Zone at their joint facility at London Heathrow Airport for the handling and storage of pharmaceutical shipments.

Sartorius begins building a new, EUR 30-million (US$35.2 million) Cell Culture Technology Center in Ulm, Germany.

Kraig Biocraft Laboratories, a developer of spider silk-based fibers, will open a new facility in Michigan to combine its research and production efforts.

A grant from the Bill & Melinda Gates Foundation will advance PnuVax’s pneumonia vaccine’s clinical development and biomanufacturing scale-up using a low-cost manufacturing approach.
Manufacturers introduce innovations in glass and plastic packaging for injectables.
Detecting viral contaminants in biologic-based medicines-and identifying their source-requires a holistic testing approach.

Process analytical testing for biopharmaceuticals requires enhanced methods due to complex bioprocesses.

This study outlines methods for an alternative protein-polishing process for challenging proteins.
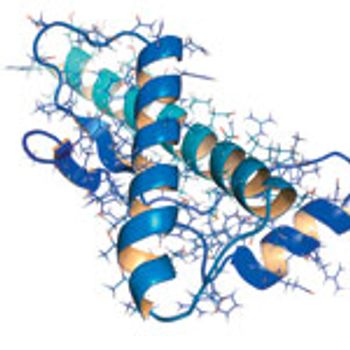
. This study is an attempt to produce a fusion protein by binding the fragment NT-gp96 in upstream of sequence of the N terminal fragment (NT300) of the NS5B gene in an expression vector.

DoKaSch Temperature Solutions, a Germany-based provider of climate-controlled air cargo containers, announced that its Opticooler air cargo containers are now available in the United States.

Eurofins Scientific has opened a £4-million (US$5-million) pharmaceutical chemistry and microbiology facility in Livingston, Scotland, to support the company’s product and water testing businesses in the UK.

Nemera is now authorized to handle, assemble, sterilize, and store pharmaceutical drugs and medicinal products for autoinjector combination products at its facility in Neuenburg, Germany.

KBI Biopharma, a biopharmaceutical contract development and manufacturing organization, will manufacture an antibody and a fusion protein developed by Heat Biologics’ subsidiary.

Fresenius Kabi broke ground on a previously announced $250 million expansion of its Melrose Park, IL, manufacturing facility.