
Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.

Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.
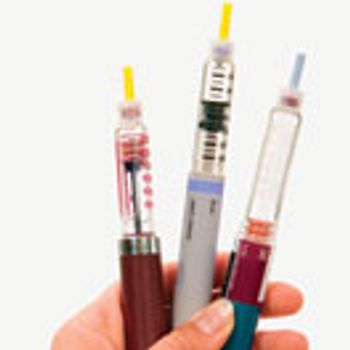
Siliconization is a key process step in the manufacturing of prefilled syringe systems.

The biotechnology company has entered a contract with the United States Army to develop custom recombinant spider silk for protective textile applications.

The vaccine is approved for the prevention of shingles in adult patients aged 50 years and older.

As part of the $900-million deal, Incyte will pay $150 million upfront to develop and commercialize an anti-PD-1 drug candidate from biopharmaceutical company, MacroGenics.

AbbVie will pay a $205-million upfront payment and have the option to develop and commercialize two antibody targets globally.

Inovio has reported results from a study with non-human primates that showed 100% effectiveness with a DNA vaccine the company is developing with the US Army.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.

The M&A advisory firm has developed an interactive map of manufacturing sites to give insight into the market size of global manufacturing.

ABEC, an equipment and engineering company, will provide a custom-made, single-use bioreactor to custom manufacturing firm, Emergent, for its Maryland manufacturing facility.

How soon could the pharmaceutical industry see a widespread adoption of emerging technologies that are poised to shape its future?

The biopharmaceutical company has received a $4.2-million grant from the Bill & Melinda Gates Foundation to invest in the development of new treatments for Enterotoxigenic Escherichia coli infections, a bacterial cause of diarrhea in the developing world.

The API manufacturer has announced that it has completed the expansion of large-scale manufacturing capabilities at its Charles City, Iowa site.

The $72-million investment, part of a larger $850-million investment into its US operations, will allow the drugmaker to replace an outdated insulin vial-filling line and to upgrade technology at its Indianapolis manufacturing plant.

The clinical-stage biopharmaceutical company has announced the opening of a new gene therapy manufacturing facility in Modiin, Israel, which is anticipated to be the production site of the company’s lead cancer drug candidate.

The biotechnology company has officially opened its new Michigan research and production center for the production of spider silk-based fibers, as well as for the company’s polymer research and development program.

The European Commission and EMA have created an action plan to address challenges identified by stakeholders involved in developing advanced therapies.

This approval marks the second gene therapy to be approved by FDA and the first to be approved for certain types of non-Hodgkin lymphoma.

The companies aim to use CureVac’s proprietary messenger RNA technology to develop and commercialize up to five potential cancer vaccine products.

SCHOTT began production of glass ampoules, vials, and cartridges at a new facility at the SCHOTT Xinkang headquarters in Zhejiang, China.

The two companies announced their collaboration for the production and development of cancer vaccines.

The partnership and the formation of the institute intend to bring together industry, academia, and regulators to tackle challenges and provide solutions for continuous manufacturing.

Almac Group’s Almac Pod, a temperature-controlled shipping solution service for biologics and other temperature-sensitive products, is available in the United States.

The vaccine is a non-live, recombinant subunit vaccine that combines an antigen and an adjuvant system to trigger a targeted and long-lasting immune response to the shingle-causing virus.

The companies will collaborate to produce and improve the recombinant properties of Aethlon’s Hemopurifier blood purification device.