
Under this partnership, Johnson & Johnson and BARDA will focus on the advanced development of a small-molecule drug and vaccine for the pandemic flu.

Under this partnership, Johnson & Johnson and BARDA will focus on the advanced development of a small-molecule drug and vaccine for the pandemic flu.

The new 10,000 ft2 of laboratories will be dedicated to the company’s monoclonal antibody platform, which enables rapid access to development and manufacturing capacity.

Bormioli Rocco Pharma, an Italian supplier of glass and plastic packaging, has launched its Delta-molded, type 1 glass vials in North America.

The authors review methods for generating monoclonal antibodies for research and development.
Traditional planar culture formats are being superceded by microcarriers for large-scale cell therapy manufacturing.
A case study demonstrates that affinity chromatography can offer efficiency and scalability for gene therapy manufacturing using viral vectors.
Consider automation early in the rollout of clinical translation and scale up of clinical-trial protocols.
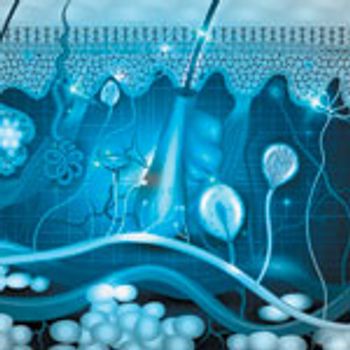
Using a hollow microstructured transdermal system to deliver vaccine directly to the dermis.

The development of emerging therapies poses unique manufacturing and formulation challenges for drug developers as candidates like cell and gene therapies advance through the pipeline.

Focused around Purdue University’s LyoHUB, a new blueprint aims to bring innovation to equipment and processes. One goal? Continuous freeze drying.

Shingrix represents a new, possibly better alternative to existing treatments.

The agency is looking for industry input on best practices for continuous manufacturing.

Catalent and US WorldMeds have entered into an agreement for the commercial manufacture of lofexidine, a drug used to alleviate opioid withdrawal symptoms.

Modeling tools help process engineers optimize a biopharmaceutical facility’s capacity.

Single-use and single-pass TFF devices are facilitating advances in biopharma manufacturing.

Choosing a suitable material for fill/finish containers begins during the product development stage.

Internet of Things, advanced analytics, and blockchain solutions such as smart contracts promise to give manufacturers more control over products and supply chains.
The control of biologics microbiological impurities, contaminants, and mimetics is evolving.
Protecting against microbiological contaminationover the whole manufacturing process grows increasingly important.

The International Society for Pharmaceutical Engineering announced the release of ISPE GAMP Good Practice Guide: IT Infrastructure Control and Compliance (Second Edition).

An FDA evaluation concluded that Corden Pharma Latina’s corrective actions addressed the concerns in an FDA warning letter.

The International Society of Automation (ISA) and Siemens entered a global partnership to increase awareness of industrial cybersecurity needs and standards.

FDA approves Novartis’ CAR-T therapy, marking the first time a cell therapy based on gene transfer has been approved in the United States for any indication.

The acquisition of Protein Sciences, a vaccines biotechnology company, strengthens Sanofi’s influenza vaccines portfolio.

FDA halts unproved stem-cell cancer treatments administered to California patients, which was derived from a commercially unavailable military-grade vaccine.