
Microchannels show potential benefits for inertial cell sorting and for introducing genetic material into cells.

Microchannels show potential benefits for inertial cell sorting and for introducing genetic material into cells.

The authors review the technologies that may help bioprocessing become a truly continuous operation and present case studies that could contribute to the integration of upstream and downstream platforms.

A modular cell-culture platform demonstrates accelerated process development.
The authors provide application data to support the use of SEC beyond small-scale operations.

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

The pharma company revealed in a fourth quarter call that it will improve its cell-culture capabilities by focusing on the use of naïve, highly proliferative cells to manufacture its CAR-T drug candidate.
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Experts in the field share some best practices for optimizing process economics in biomanufacturing.
The authors describe the ways in which manufacturers can mitigate the risks related to the integrity of recombinant transgenes expressed in CHO cells.
The necessity to detach cells from a culture substrate during cell harvesting remains one of the most challenging steps in a cell-culture process.

Collaborative efforts are underway between suppliers and drug manufacturers to address raw material variability.

Microbial models offer some exciting production alternatives.

Miniature bioreactors add value by reducing validation efforts.
The authors review the status of expression of antibodies in microbial hosts and present the recent advances in the production of aglycosylated antibodies in bacteria.
Rapid methods to test CAR-T therapies for potential contamination are on the horizon.

The authors describe the impact of the knocking of the pgi gene of the wild type MG1655 strain on the growth kinetics of plasmid-free and plasmid-bearing cells.
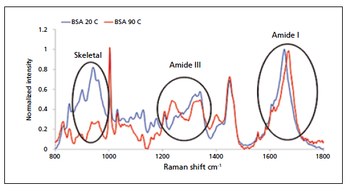
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.

Novasep is building a new synthesis laboratory and adding capacity for kilogram-scale batches of synthetic molecules that are needed for biological testing and preclinical trials, at its Pennsylvania, US facility.

Takeda Pharmaceuticals announced the acquisition of a biopharmaceuticals manufacturing plant in Minnesota.

FDA discusses a new program that allows pharmaceutical companies to submit proposals for new manufacturing technology.
In semiconductor manufacturing, for example, a thorough understanding of process variation allows companies to manufacture circuits with billions of transistors at high yields. These variations are translated into a set of design rules, which help ensure that designs will be manufactured successfully and meet safety and other regulatory requirements.
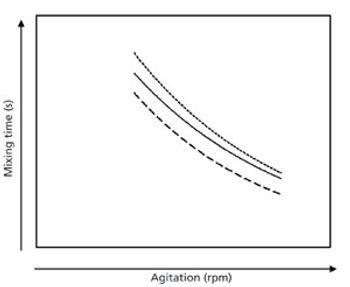
The authors conclude that miniature bioreactors can adequately predict the cell culture kinetics in scaled-up reactors using equal mixing times.

Boehringer Ingelheim announced it will establish a new biopharmaceutical production facility in Vienna.

Biogen, Genentech, Johnson & Johnson, Novartis, and Patheon publicize their support for action on climate change.