
A new facility type integrates next-generation mobile cleanroom systems.

A new facility type integrates next-generation mobile cleanroom systems.
The use of therapeutic vaccines presents a new way to manage diseases, such as cancer and sexually transmitted diseases.

Alternatives to time-consuming, error-prone operations promise to reduce vaccine manufacturing costs and improve facility flexibility.

ADC Bio experts warn of impending problems in the ADC pipeline with millions wasted in development costs.

Company completes first successful run of what is believed to be the largest synthesis-scale done for oligonucleotide APIs.

The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.

CDMO Celonic acquired a biomanufacturing facility in Heidelberg, Germany from Glycotope.

The companies will collaborate to develop and manufacture Grid’s lead therapeutic candidate for the treatment of solid tumors.
Connectors are a critical element in the process optimization of single-use bioprocessing systems.
Despite the disappointing therapeutic performance of ADCs thus far, the pipeline still boasts promising prospects.

Cole Parmer's Masterflex L/S Cytoflow pump head is suited for use in biopharma and microbiology applications.
Anna Kireieva/Shutterstock.comSpeed to market is essential in the biopharmaceutical industry today.
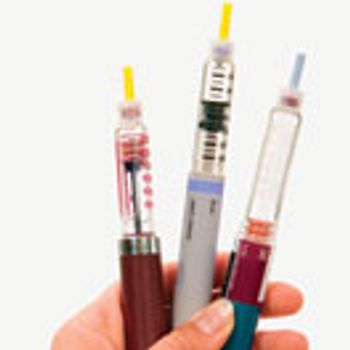
Siliconization is a key process step in the manufacturing of prefilled syringe systems.

The biotechnology company has entered a contract with the United States Army to develop custom recombinant spider silk for protective textile applications.

As part of the $900-million deal, Incyte will pay $150 million upfront to develop and commercialize an anti-PD-1 drug candidate from biopharmaceutical company, MacroGenics.

AbbVie will pay a $205-million upfront payment and have the option to develop and commercialize two antibody targets globally.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.

ABEC, an equipment and engineering company, will provide a custom-made, single-use bioreactor to custom manufacturing firm, Emergent, for its Maryland manufacturing facility.

The biopharmaceutical company has received a $4.2-million grant from the Bill & Melinda Gates Foundation to invest in the development of new treatments for Enterotoxigenic Escherichia coli infections, a bacterial cause of diarrhea in the developing world.

The $72-million investment, part of a larger $850-million investment into its US operations, will allow the drugmaker to replace an outdated insulin vial-filling line and to upgrade technology at its Indianapolis manufacturing plant.

The clinical-stage biopharmaceutical company has announced the opening of a new gene therapy manufacturing facility in Modiin, Israel, which is anticipated to be the production site of the company’s lead cancer drug candidate.

This approval marks the second gene therapy to be approved by FDA and the first to be approved for certain types of non-Hodgkin lymphoma.

The companies aim to use CureVac’s proprietary messenger RNA technology to develop and commercialize up to five potential cancer vaccine products.

The partnership and the formation of the institute intend to bring together industry, academia, and regulators to tackle challenges and provide solutions for continuous manufacturing.

The companies will collaborate to produce and improve the recombinant properties of Aethlon’s Hemopurifier blood purification device.