
The agreement gives IncoCell Tianjin, a wholly-owned subsidiary of China-based Boyalife Group, access to Cesca’s celluar processing contract development and manufacturing services.

The agreement gives IncoCell Tianjin, a wholly-owned subsidiary of China-based Boyalife Group, access to Cesca’s celluar processing contract development and manufacturing services.

Hitachi will manufacture regenerative medicines developed by Daiichi Sankyo and SanBio Group.

The collaboration between the two companies aims to finish all necessary work needed to file for a first-in-human study by early 2019.

The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

Sartorius has delivered to ABL’s Strasbourg facility, a GMP viral vector manufacturing package solution that includes single-use bioreactors and an automation platform for normal flow filtration, tangential filtration, and mixing.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

Under this agreement, the companies will develop in parallel an antibody drug candidate and cell lines for other potential candidates.

GlobalData reports the need to shift away from egg-based manufacturing of vaccines in light of influenza-related deaths.
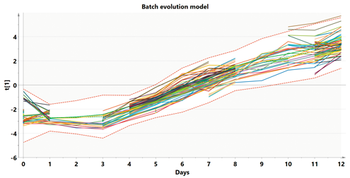
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

Spectroscopic tools present an alternative method for reliable at-line process monitoring and control.

Biologic new molecular entities (NMEs) accounted for 26% of total NME approvals in 2017.

The contract development and manufacturing organization has entered it first manufacturing contract worth $148 million for its recently completed Plant 3 facility.

The new training center, the Jefferson Institute for Bioprocessing, will prepare engineering students and industry professionals for the field of biologics manufacturing.

The study suggests that circumventing evolution in cell factories can enable the commercialization of new biobased chemicals to large-scale.

Ompi EZ-fill vials and Daikyo Seiko PLASCAP press-fit closures are a confirmed product set for use with Vanrx Pharmasystems' Aseptic Filling Workcells.

Ferring Pharmaceuticals will expand its biologics capabilities at its headquarters and manufacturing site in Saint-Prex, Switzerland.

Unlike current approaches where bioconjugation is typically done following the manufacture of the monoclonal antibody and the cytotoxic drug, the new method begins with the antibody supernatants and eliminates the need for extensive chromatographic purification.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.

Amgen plans to invest approximately $300 million in a new biomanufacturing plant in the United States.

A collaboration between The Centre for Process Innovation and The Roslin Institute aims to develop commercially viable and scalable methods of producing biologics using transgenic animals.

The Flexicon PF7 peristaltic tabletop aseptic liquid-filling machine from Watson-Marlow Fluid Technology Group is suitable for GMP-regulated biotechnology and pharmaceutical operations.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.
As closure integrity testing moves from a probabilistic to a deterministic basis, designs are promoting improved control and reduced operator contact.

Greater clarity on the application of existing regulations will accelerate development of cell and gene therapies.
Single-use technologies are starting to gain ground as capacity needs change, but industrywide adoption remains low.