
Under terms of the agreement, Zymeworks could earn up to $164 million per successful drug candidate.

Under terms of the agreement, Zymeworks could earn up to $164 million per successful drug candidate.

Catalent announced that it would partner with Mitsubishi Gas Chemical Company, and its subsidiary MGC Pharma, to promote GPEx technology, a high-titer vector for stable mammalian cell lines.

Sanofi will tap into Boehringer Ingelheim’s therapeutic monoclonal antibody manufacturing capabilities.

Single-use components aid efficiency in automated personalized therapy manufacturing.

Improving efficiency, value chain, quality, and protein complexity with advanced bioprocess development.

The partnership will focus on the discovery of antibodies against proteins that are not easily purified in functional form.

Roche will use Dutalys’ DutaMab technology for the engineering of bispecific therapeutic antibodies.

Roche will use Dutalys’ DutaMab technology for the engineering of bispecific therapeutic antibodies.Roche Acquires Bispecific Antibody Developer Dutalys

Biopharma continues to adopt single-use technologies, but also seeks more innovation and improvements in products.

Representatives from industry supplier companies address innovations, reliability, barriers to adoption, and the role of singIe-use bioreactors in bioprocessing.

A dynamic market, industry consolidation, and demand fluctuations lead to a mixed picture of pricing results.

Representatives of contract service organizations discuss technology trends with BioPharm International.

Mike Jenkins, general manager of Catalent Biologics discusses the evolving landscape of the biologics market and hurdles faced in the development and manufacture of these innovative products.

The EMA's Committee for Medicinal Products for Human Use has recommended granting of marketing authorizations for the first two monoclonal antibody biosimilars.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

A Q&A with Rick Hancock, president of Althea Technologies. This article contains bonus online material.
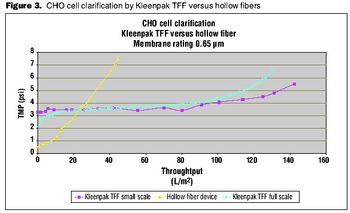
Choosing the right tools to enhance the process.
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.
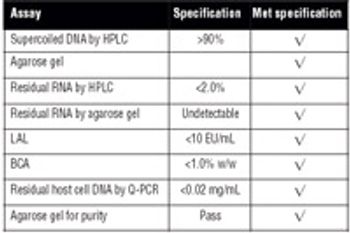
Recombinant protein and plasmid DNA production using microbial expression systems is the cornerstone of many biologics manufacturing processes. HCD methods are commonly used for these processes because of the advantages they provide.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

The United States Pharmacopeia (USP, Rockville, MD, www.usp.org) and the UK's National Institute for Biological Standards and Control (NIBSC, Hertfordshire, UK, www.nibsc.ac.uk) are seeking participants in a study of analytical methods used by the industry to characterize and quantify oligosaccharides.

The sugars used to stabilize lyophilized proteins often have not been subjected to appropriate cGMP standards.

The type of reactive moiety controls the site and stability of the covalent link and also the total number of PEGylation sites on a given protein.