
PSC Investments announces the acquisition of a high potency, sterile fill/finish pharmaceutical manufacturing facility.

PSC Investments announces the acquisition of a high potency, sterile fill/finish pharmaceutical manufacturing facility.

Cubist Pharmaceuticals voluntarily recalls certain lots of CUBICIN 500 mg in 10 mL single-use vials because of the presence of particulate matter.

Unique Pharmaceuticals has issued a voluntary recall of sterile compounded preparations, but aired concerns about FDA?s recall demand.

FDA issues a Form 483 to Wockhardt for quality issues at Morton Grove, IL facility.

Clinical Syringe Packages Increase Productivity

Integrated Single-Use Sterile and SIP Connector Provide Flexibility

Aseptic Valve Range Increases Sterility

New injection-delivery systems with multiple closure points pose challenges for container closure integrity testing.

A novel approach to sterile drug product manufacturing uses a single-use assembly in a multi-product final filling suite with isolator technology.

Tandem Diabetes Care expands its voluntary recall of select lots of insulin cartridges used with t:slim insulin pump.

Drug Quality and Security Act gives FDA authority over compounding pharmacies.

Wolfgang Weikmann of Vetter Pharma discusses the implementation of quality by design in sterile manufacturing.

ATMI has invested in sterile-connector and sterile-fill technology developed by Medical Instill Technologies.

FDA receives adverse event reports related to calcium gluconate infusions.

Vetter has ready-to-submit documentation for this service in Common Technical Document (CTD) formats for the US, Europe and Japan.

Third compounding pharmacy recalls products due to FDA inspection.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

Ray O'Connor, an operations consultant with NIBRT, addresses aseptic processing, including how to avoid contamination, and cleanroom best practices. Posted May 2012.

The International Society for Pharmaceutical Engineering (ISPE) will soon publish an update for its guide to sterile-product manufacturing facilities. The new publication will replace the original guide, ISPE Baseline Guide: Sterile-Product Manufacturing Facilities, and contain practical information about technological advances in sterile manufacturing.
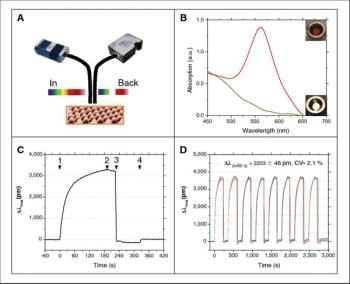
Use it label-free, or add labels to detect contaminants in solution.

How this Big Pharma company successfully implemented disposable technologies in its manufacturing plant.

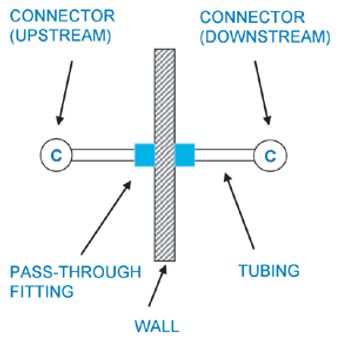
Many factors must be considered when choosing a sterile connector for a given process.

The European Medicines Agency (EMEA) has granted license approval to Bayer HealthCare LLC (Bayer, Berkeley, CA) for its new sterile filling facility on its Berkeley, CA, campus.

The industry needs to open up to validation failures.