
The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.

The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.
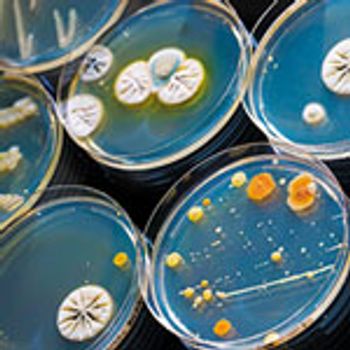
Microbial identity data can be critical for determining contamination sources.
Unprocessed bulk material harvested directly from the bioreactor should be tested for contamination prior to downstream processing.

Bioconjugation requires aseptic manufacturing and containment for cytotoxic payloads.

A matrix of multi-functional cleanrooms can be adapted for launching products.
This article will explore the requirements for media and supplements needed to maintain newer cell lines, such as those based on human cells and fungal cells.

Kimberly-Clark Professional added the Kimtech A5 Sterile Integrated Hood and Mask XL for head shapes, sizes, and hairstyles that pose a challenge to standard aseptic gowning for cleanroom operators.
This article explores the use of single-use mixing technology in a detergent-based virus inactivation step during a monoclonal antibody production process.

The sterile-manufacturing contract development manufacturing organization is approved by FDA for viral vector manufacturing fill/finish processing at its biologics facility in Scotland, UK.

The company is increasing manufacturing capacity at its Copenhagen, Denmark facility with the addition of six new bioreactors.

Watson-Marlow Fluid Technology Group added a new actuator suitable for applications where reduced weight is a concern.

Automation can improve many aspects of bioprocessing, but several hurdles must be overcome before the full range of benefits can be realized.

Viral vaccines and viral vectors used in biotherapeutic applications carry the risk of microbial contamination, which must be addressed.

The US Pharmacopeial Convention (USP) is developing a new chapter for rapid sterility testing of short-life products based on the recommendations of a panel of experts and stakeholders.

Pfizer will invest nearly half a billion dollars to build a sterile injectable facility in Michigan.

Understanding differences in bioreactor lifecycle, design space, and product platforms is important for selecting a bioreactor type.

Single-use technologies have become increasingly prevalent in final fill/finish operations for biologics.

Vetter anounced the Open Innovation Challenge to examine the applicability of digital trends to injection systems.

RGtimeline/Shutterstock.comParenteral product quality is improving.
Developments and investments in single-use systems advance upstream biomanufacturing.

The author reviews operative options for the implementation of a quality oversight and how companies can benefit from this function from a regulatory perspective.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

The company is voluntarily recalling three lots of Labetalol Hydrochloride Injection, USP, 100 mg/20 mL Vial and one lot of Labetalol Hydrochloride Injection, USP, Novaplus because of the potential of cracked glass at the rim of the vials.

Ompi EZ-fill vials and Daikyo Seiko PLASCAP press-fit closures are a confirmed product set for use with Vanrx Pharmasystems' Aseptic Filling Workcells.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.