
Manufacturing, Aseptic Processing
Latest News




The US Pharmacopeia (Rockville, MD, USP, www.usp.org) recently announced that the implementation period for its USP–NF general notices statement requiring all manufacturers to conform to recently revised residual solvent standards in General Chapter <467> has been extended from July 1, 2007 to July 1, 2008.

Membrane-based chromatography technologies sometimes offer advantages over resin-based technologies.

Although contaminants and other parameters may be main causes of filter breakdown, some nanofilters still remove viruses at high Log Reduction Value (LRV).

The HSV-1 and HVP-2 titers were determined by the inoculation of test solutions into Vero cell cultures and calculated using the Reed M?ench method.

NovAseptic mixers from Millipore (www.millipore.com/bioprocess) are designed for a variety of mixing applications in the pharmaceutical and biotechnology industries and are magnetically driven, which minimizes contamination risk.
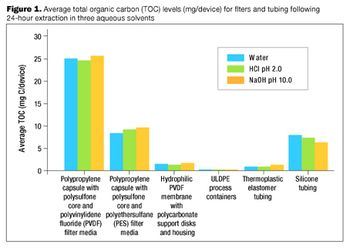
The many benefits of disposable technologies, such as significant savings in time, labor and capital, as well as ease of scalability and flexibility, have led to the growing trend of adopting disposable technologies in bioprocess manufacturing processes.
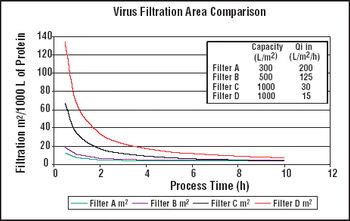
It is important to ensure that flow decay during processing is comparable to that observed during retention studies.

Steam traps are part of a steam-in-place system. The current design allots 18 in. of vertical leg for condensate backup. A design with a sensitive bellows has been proven in laboratory tests to need only 6 in. of vertical leg during the 15 min. of 121?C sterilization. Loads of 1 to 27 lb/h are covered by the capability of the new trap, equivalent to required steam for vessels 20 to 40,000 L.

Disposables require less space than conventional equipment, and they can be assembled offsite into complete process trains.

A symposium at the AAPS National Biotechnology Conference in Boston, June 19-22, 2006, addressed key concerns and new developments in manufacturing biologic products in a sterile environment.

On August 12, 2003, Johnson & Johnson began recalling certain batches of its anemia drug, Eprex (epoetin alfa, sold as Procrit in the US), in most countries outside of the United States.

Disposables can be used for media preparation, clarification, filling in downstream processes . . .

In the pharmaceutical industry, ultrafiltration (UF) membranes are used extensively in the downstream purification of recombinant proteins or monoclonal antibodies. However, the fouling of membranes after a unit operation?especially when recombinant proteins or monoclonal antibodies are highly concentrated?is a common problem. Typically, normalized water permeability (NWP) of a membrane can be reduced to about 20 percent of its original permeability at the end of an ultrafiltration-diafiltration (UF-DF) operation.

Before designing cleaning procedures, it's vital to know all physical and chemical characteristics of the product ingredients.

