
The contract manufacturing company has completed construction on a new aseptic fill/finish facility in Wuxi, China.

The contract manufacturing company has completed construction on a new aseptic fill/finish facility in Wuxi, China.
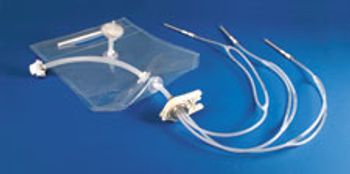
Sterile-molded filling assemblies from AdvantaPure are suited for single-use vial and syringe filling.

The recall was initiated due to a product complaint in which white particulate matter, identified as mold, was discovered in a flexible bag from one batch.

Alternatives to time-consuming, error-prone operations promise to reduce vaccine manufacturing costs and improve facility flexibility.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.

The company is investing EUR 170 million (US$201 million) to expand its vaccine manufacturing site in France for its newest influenza vaccine.

This study outlines methods for an alternative protein-polishing process for challenging proteins.

Fresenius Kabi broke ground on a previously announced $250 million expansion of its Melrose Park, IL, manufacturing facility.

Choosing a suitable material for fill/finish containers begins during the product development stage.
The control of biologics microbiological impurities, contaminants, and mimetics is evolving.
Protecting against microbiological contaminationover the whole manufacturing process grows increasingly important.

Ajinomoto Althea’s Optima VFVM 7000 aseptic fill/finish line supports a range of drug substance APIs.

FDA sent a warning letter to Sage Products, Inc. regarding CGMP failures involving test methods, sampling, and sterilization procedures.

Fagron Sterile Services has voluntarily recalled three lots of Succinylcholine Chloride 20mg/mL 5mL syringe to the hospital/clinic level.

The company has voluntarily recalled Clindamycin Injection USP ADD-Vantage Vials to the hospital/retail level because of a lack of sterility assurance.

FDA sent a warning letter to drug compounder DCA, Inc. dba Beacon Prescriptions for failing to ensure sanitary conditions.

FDA sent a warning letter to Pharmaceutic Labs, LLC for deficiencies in producing sterile drugs.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.

Avella issued a nationwide recall of sterile products produced at the Advanced Pharma Houston location due to inaccurate labeling.

FDA sent a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found deviations in the facility’s aseptic processes.

The company has voluntarily recalled all lots of of human chorionic gonadotropin because of a lack of sterility assurance.

The agency sent a warning letter to Resonance Laboratories Pvt. Ltd. after an inspection found possible contamination problems.

Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.