
Unfortunately, once circuits are commissioned and validated, optimizing - or even adjusting - them is difficult and rarely done because of the time and effort required for revalidation.


Unfortunately, once circuits are commissioned and validated, optimizing - or even adjusting - them is difficult and rarely done because of the time and effort required for revalidation.

Manufacturing changes - such as changes in formulation or source material - can impact a product’s immunogenicity.

In order to institute a GxP mindset across the organization, support and respect for quality systems should come from the top down.
What are current good manufacturing practices (cGMPs)? Where did they come from? What are the actual "practices" described in the Code of Federal Regulations, 21 CFR. If you are new to the pharmaceutical or biotechnology industries, you may enter your first "GMP Training" session without much context or perspective. A set of arcane rules is presented; you were never taught these in science classes.

Good manufacturing practices (GMPs) are manufacturing guidelines for ensuring the safety and efficacy of drug products and medical devices. The GMPs are legal regulations, based on the United States Food, Drug and Cosmetic Act. But, why do we need the GMPs? Shouldn't we, as knowledgeable individuals, groups, and companies, be able to figure out how to produce drugs and devices that are safe and effective?

GMP is the acronym for Good Manufacturing Practice. The GMPs represent a set of regulations that were promulgated as a final rule by FDA in 1978 and intended to ensure the safety and efficacy of the nation's drug products. The GMPs, as we know them today, are the result of over a century of actions by industry and reactions by government and consumer groups to bring guidance and controls to the food and drug industry, resulting in a safe supply of food and medicines.

There continues to be much interest within industry and FDA about the future of the GMPs. Discussion groups have been spawned within professional organizations and at FDA to reevaluate the aging GMPs and associated guidance documents to ensure that the government does not impede technological progress, focuses its resources effectively, and upholds its mandate to protect the public.
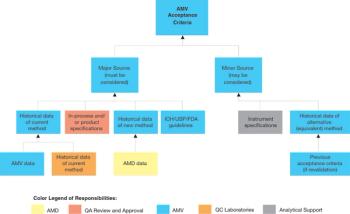
When replacing an existing method, it may be necessary to compensate for a significant difference between the current and new method by adjusting the specifications.

The relationship between "valid" or "suitable and validated" is often overlooked, but there is a high price when "validated" test systems are simply inappropriate.
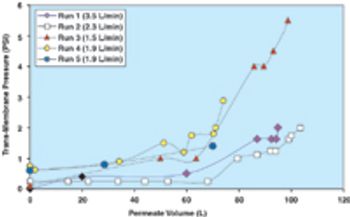
Filtration is one of the most commonly used unit operations in the manufacturing of biopharmaceuticals. This is the second part of the fourth article in the "Elements of Biopharmaceutical Production" series. In this second segment, Manoj Menon and Frank Riske present an approach for the development and optimization of a TFF application, followed by a contribution from Jennifer Campbell and Elizabeth Goodrich reviewing key issues involved in validation of a TFF step.
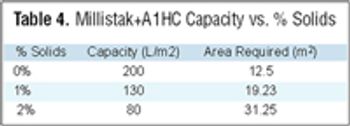
Filtration is one of the most commonly used unit operations in biopharmaceutical manufacturing. Available formats include direct or normal flow filtration (NFF) and cross or tangential flow filtration (TFF). These methods are used for sterilization and virus filtration, depth filtration or ultrafiltration, and diafiltration applications. Some common objectives include:

Although there are many differences between the industries, especially related to regulatory requirements, there are enough similarities that the future of biopharmaceuticals with respect to contract manufacturing might look much like the semiconductor industry.

Spreadsheet calculations are popular in all kinds of businesses. A macro is a set of commands that can be embedded in a document to add functionality to standard programs and to automated processes.

The European Union requires final container testing of US-manufactured biopharmaceutical products to be performed in Europe for release into the European market. Similarly, but less strictly enforced, the US requires final container testing in the US for European-manufactured biopharmaceutical products before release.

It is not likely that you will ever personally design and build a cleanroom; however, you may be responsible for the care and upkeep of many.
Networks are part of the compliance picture. Recent FDA warning letters show the agency considers network monitoring and qualification a necessary part of maintaining the security and integrity of electronic records.

The FDA rule on electronic signatures and electronic records was issued in 1997, but the details of implementation are still being debated. The 2003 FDA guidance redefines the scope of 21 CFR Part 11. Understanding which records now fall under the scope of the rule can help you begin implementing your compliance plan.

The FDA?s risk-based approach to pharmaceutical cGMPs applies to 21 CFR Part 11 enforcement as well. Understanding different methodologies for assessing and managing risk will help you develop and begin to implement a compliance plan.

Protecting the integrity of data is a challenge of 21 CFR Part 11 compliance. Integrity requires records to be complete, intact, and maintained in their original context ? associated with the procedures which were used to create the data.

Bringing different laboratory instruments into compliance takes planning. The key strengths and weaknesses of different levels of control and feedback for analytical instruments and data transfer systems are highlighted in this article.

This article focuses on the front end of qualifying a new raw material from a given supplier. Once qualified, this status must be maintained by periodic review and requalification.

Training programs do not have to be complex to be successful. There are basic elements, however, that all must have to meet FDA requirements and ensure that employees have the knowledge and skills to maintain high quality standards.

Biotech companies are among the most intensive instrumentation users of any FDA-regulated business. Because of the complex nature of these companies' research and manufacturing, they typically have a larger number of instruments per employee.

Good laboratory practice is the central dogma of all laboratory research and investigation - it's your commitment to regulators - and poor laboratory controls are a common cause of 483 observations and preapproval inspection failures.

Supplementing your existing staff with experienced contractors when your process is ready for validation can help you avoid common validation mishaps - if you know the ingredients of successful project management.