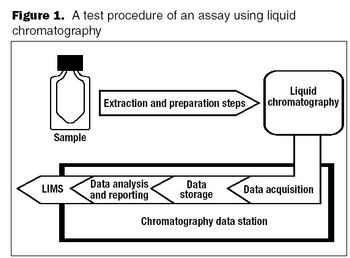
The outsourced service provider should be considered an extension of your own laboratory.

The outsourced service provider should be considered an extension of your own laboratory.

After a strategic evaluation of what activities to outsource, sponsor companies should follow key guidelines for selecting and auditing a provider and preparing quality agreements.
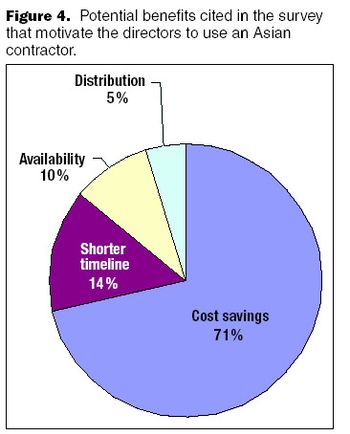
When a biopharmaceutical company pursues an outsourcing strategy, the choice of a contractor is a critical and strategic decision.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.

A case study investigated the root cause of failures in sterile filtration by evaluating the effects and interactions of four operating parameters.

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

Now is a good time for companies to know their suppliers well.

The supply base for preclinical and clinical development services continues to expand in China.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

More informed submissions may lead to regulatory flexibility for postapproval changes.
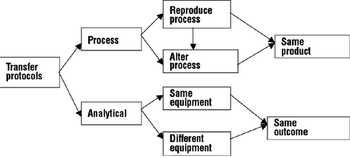
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.
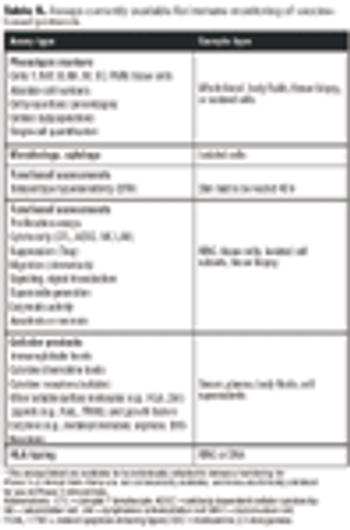
Emerging therapies pose challenges for standardizing QC.

The expanding globalization of the industry poses a challenge for international enforcement.

The cause of the heparin crisis is still unknown. We do know, however, that the FDA is severely underfunded.

Need a standard to follow? Just ask, What would Genentech do?

Both innovator and generics companies are using analytics to support comparability arguments.

Best practices from Big Biotech, including how to handle new product introductions.
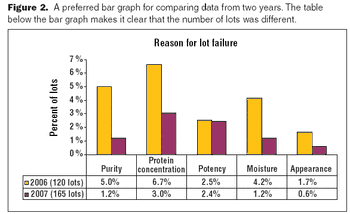
The key to a good graphical presentation is to select the method that best fits the data.

The new year begins on a note of optimism. A major breakthrough in stem cell research promises to open the door to new biomedical research opportunities. The drawn-out Congressional debate over user-fee reauthorization and drug safety regulation is over, and most parties seem satisfied with resulting compromises. The vaccine industry is experiencing a resurgence after years in the doldrums, with important new vaccines on the market and more under development. And unlike many previous years, the US Food and Drug Administration (FDA) had a confirmed commissioner for all of 2007 and relatively stable leadership.

Design space concepts are key to a successful technology transfer.

How the authors used design of experiments and quality by design principles to develop a hydrophobic interaction chromatography step.
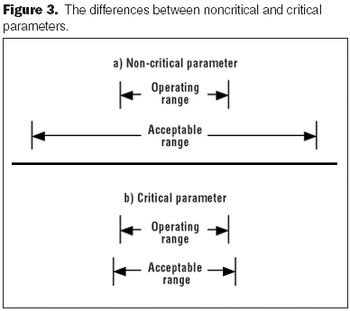
Developing a process validation strategy early in clinical development is critical for a successful validation program.

Many companies acknowledged that Western regulatory standards are tougher than those in China.

The personalized medicine bandwagon is on a roll, offering a new model for calculating reimbursement of high-cost biotech therapies. Strategies for identifying patients who will respond to a certain therapy, as well as those most likely to suffer adverse events, promise to improve healthcare quality while eliminating waste and inappropriate spending. Interventions based on individual genetic characteristics may have limited sales, but support higher prices and less costly clinical research methods.

Congress postpones debate on follow-on biologics while adopting new policies likely to reshape drug development