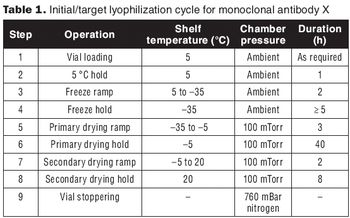
Develop a relevant design space without full factorial DoE.

Develop a relevant design space without full factorial DoE.

Regulatory relief requires that regulators trust companies to know what they are doing, and to do it-consistently.

Plant closures, product recalls prompt FDA re-evaluation of GMP enforcement efforts.
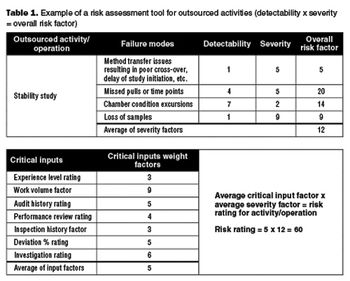
Apply risk management principles to monitor outsourced activities.
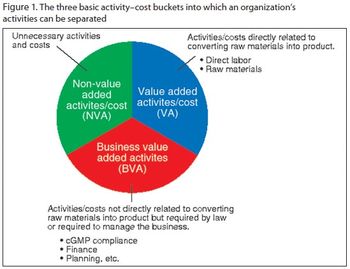
By identifying and eliminating non-value-added activities, drug manufacturers can avoid falling into the same cost-traps in the future.

A single standard should apply to all comparability exercises for biologics, be they for biosimilars or manufacturing changes.

USP is advancing efforts to develop a guidance for evaluating bioassays.

Twelve lessons of what to do and what not to do to avoid quality problems.

More information may be available on drug approvals, prices, and research to expand public understanding of regulatory policies.

International outsourcing and rising theft spur regulatory action and manufacturer oversight.

New R&D models must be tried, but it will take time to see if they work. In the meantime, a new kind of threat is on the horizon: biobetters.
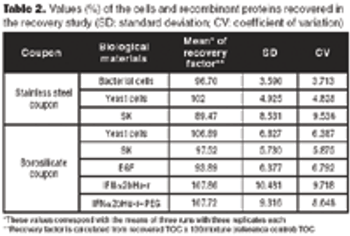
Case studies show TOC is effective for cleaning validation.

Broader benefits and biosimilars will offset hefty fees and discounts while preserving R&D incentives.

This practicum combines business elements with scientific concepts.
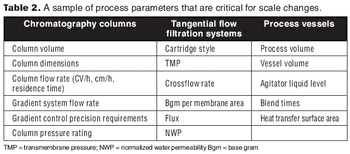
A process harmonization assessment can aid in smooth technology transfer by comparing data across equipment and sites.

Given today's challenges, traditional strategies were bound to change. Now, there is one more big threat (or opportunity?) on the horizon.

A critical GMP requirement for drug manufacturers is to validate analytical test methods to ensure that they are suitable for their intended uses. There are published guidelines available that the industry can follow for validation but there are none for qualification, which is a key step in the validation process.

The FDA is expanding postmarketing safety requirements, despite limited resources to manage these added responsibilities.

Characterizing the higher order structure (HOS) of protein drugs increases manufacturers' understanding of stability and batch-to-batch variability, and may make it possible to link variants or aggregates to safety and efficacy. Yet at the January 24 CMC Strategy Forum in Washington, DC, regulators expressed concern that methods to characterize the three-dimensional structure of proteins are not routinely applied to biotechnology products.

An effective CAPA plan provides a mechanism for responding to the unexpected.

The new Sentinel system aims to expand access to data on medical product safety and patient effects.

Trouble at Genzyme and with flu vaccine production illustrates the challenges in producing safe and potent biologics.

One might look at QbD's plodding growth and conclude that it is never going to make it to graduation.

How will implementing Quality by Design strategies affect your compliance status?

Demand for new vaccines and therapies in 2010 will be offset by concerns about drug prices and product safety.