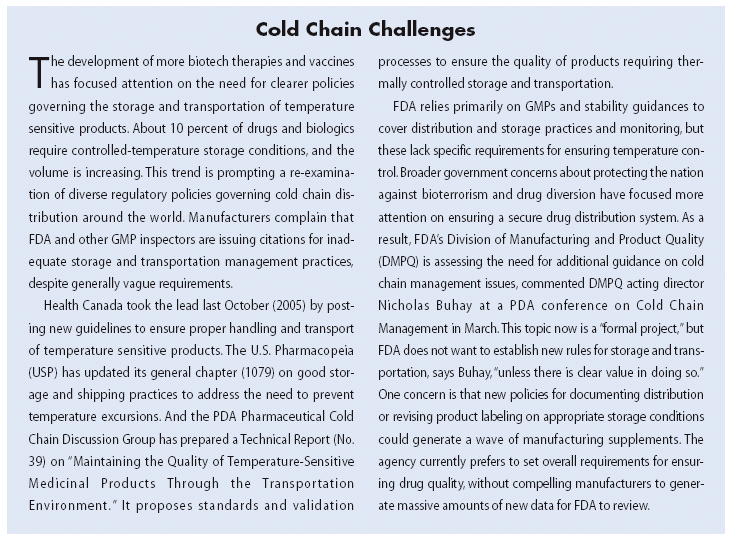
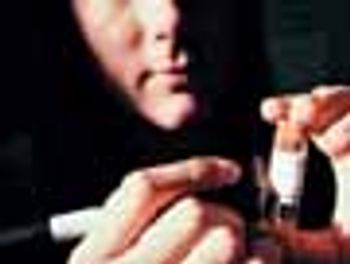
On January 17, 2006, the FDA released new regulations, effective June 1, 2006, which affect the production of most investigational drug and biologic products intended for phase 1 clinical trials. These regulations are much broader in scope than the Exploratory IND guidance released on the same day, and which apply only to low-risk, CDER-regulated clinical studies.