
What football and bioprocessing both have in common is that in both cases, success is a minimum requirement.

What football and bioprocessing both have in common is that in both cases, success is a minimum requirement.

The focus on the design space will lead to a new workspace, and will affect staff in the development, manufacturing, and quality functions.
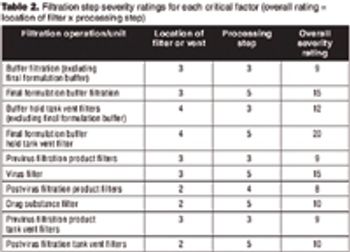
Conducting a FMEA analysis is a good first step in a risk-based approach for determining the need for a filter integrity test.

Heightened attention to product safety issues is slowing the approval process for new therapies.

It seems clear that insuring the roughly 46 million Americans who are now uninsured will increase drug sales.

Stiffer enforcement of quality standards aims to restore public confidence in agency actions.

If risk assessments only identify "the usual suspects," the process will not add much value.

ICH Q9 encourages companies to apply the concept of quality risk management. Easier said than done.

Perhaps the best way to regulate drugs is to regulate them not conservatively or liberally, but effectively.
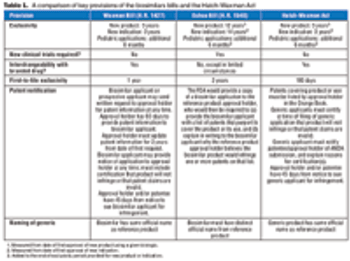
The introduction of two rival bills has intensified the long-simmering debate on biosimilars regulation in the US.

The truth is, we should have been afraid of H1N1, because the threat of a flu pandemic is real.

The FDA seeks new strategies for improving the safe use of opioids and other high-risk medicines, including erythropoiesis stimulating agents.

Agency officials promise swift action against violators of drug safety and quality regulations.

Avoiding healthcare reform is not the best option for the pharmaceutical industry.

Authorities are pushing for CE; manufacturers prefer to focus on value.
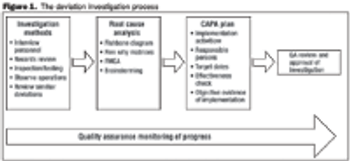
Follow a risk-based approach to maintain a state of control.

The FDA is poised to gain more authority and resources to ensure product quality.

The 45 comments submitted raised concerns about legacy products and ongoing process monitoring.
A method to evaluate the relative cleanability of new products.

At some point, will heavy investments in large, stainless-steel based facilities become a burden to US companies?

Broader transparency in product prices and payments to researchers aim to curb conflicts of interest and rationalize drug expenditures.

How change plays out will depend not only on the new Whitehouse, but on pharma leaders' ability to adapt to changing times.

Understanding the relationship between the process and CQAs.

FDA aims to regain public confidence in 2009.
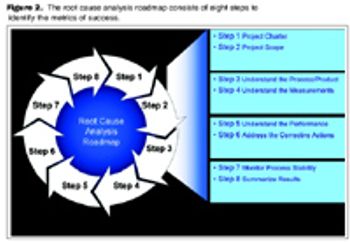
Optimize time and cost of product development by managing risk.