
Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

As more companies decouple drug substance from finished drug manufacturing operations, an integrated approach can ensure safe, reliable logistics for frozen storage and shipping.

How statistical methods and novel indices can be used to monitor and benchmark variability, to guide continuous improvement programs.
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

FDA sent a warning letter to Pharmaceutic Labs, LLC for deficiencies in producing sterile drugs.

The agency sent a warning letter to Sal Pharma for not disclosing information about API manufacturers to customers.

Can bioprocessing runs be consistently replicated in an inherently variable production environment?

New study will reveal bio/pharma practices and performance on quality issues.
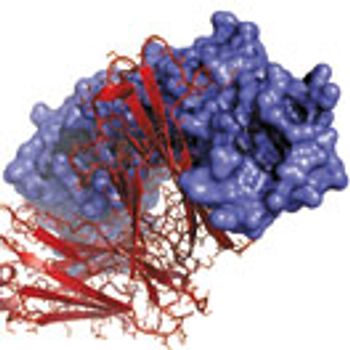
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.

At CPhI North America 2017, the US Pharmacopeial Convention will be discussing its upcoming revision and modernization of the standards for elemental and organic impurities.

Pharmaceutical Technology spoke with CPhI North America presenter Jonathan Helfgott to discuss navigating GDUFA and helpful tips for submitting successful ANDAs.

The new guide offers guidance on how to ensure data and records are complete, consistent, secure, accurate, and available throughout their lifecycle.

As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the value of internal audits and how the information gained can be applied.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

Manufacturers and regulators are working to reach consensus on the harmonization of management of postapproval changes.

FDA issued a warning letter to USV Private Limited citing CGMP violations that include data integrity and microbiological contamination issues.

The two pharmacopeias signed a Memorandum of Understanding as recognition of their collaboration for developing international science-based standards.

The agency sent a warning letter to Badrivishal Chemicals & Pharmaceuticals detailing several CGMP violations.

The agency cited Morton Grove Pharmaceuticals for inadequate quality control procedures.

The agency sent a warning letter to Chongqing Pharma Research Institute Co., Ltd. citing data integrity violations.

The Mutual Recognition Agreement will allow FDA and EU inspectors to recognize each other’s work and avoid the duplication of drug inspections.

Manufacturers face uncertainty over imports, regulatory policies, and field inspections.

Although best practices are key, advances in integrated informatics platforms and automation can make it easier to ensure data integrity and improve overall laboratory efficiency.