
Legislation to streamline drug development may get tangled up in user fee negotiations and drug pricing battles.

Legislation to streamline drug development may get tangled up in user fee negotiations and drug pricing battles.
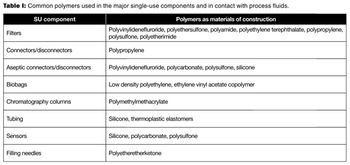
The author discusses the current best practices in technical qualification of single-use systems.

FDA’s proposed guidance for quality metrics raises questions about quantifying the tangibles and intangibles of quality culture.

Regulatory officials and industry scientists participated in a CMC Strategy Forum sponsored by CASSS in July 2015.

The agency issues guidance for companies considering registering with FDA as an outsourcing facility.

FDA issues draft guidance on dissolution testing for immediate-release solid oral dosage forms.

This article discusses cleaning validation of equipment dedicated to the production of a single API.

The broader aim of FDA's metrics initiative is to encourage quality manufacturing operations that will help avoid drug shortages.

FDA’s quality metrics draft guidance details the types of data the agency plans to request and the quality metrics it plans to calculate.

The guidance provides recommendations for submitting analytical procedures and method validation data to FDA.

FDA releases a report that analyses why some diseases are lacking treatment options.

The agency has released guidance on bioequivalence studies for asenapine, prasugrel, sitagliptin, and zonisamide.

The agency requires early notification of potential drug shortages.

The International Conference on Harmonization has moved ICH M7(R1) Addendum to 3 of the ICH process.

The European Pharmacopoeia has incorporated new provisions to encourage the use of in-process quality control methods.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to handle internal audit reports during inspections.

The US Pharmacopeial Convention hosts compliance seminar at CPhI China 2015.

The International Conference on Harmonization Steering Committee appoints Dr. Dawn Ronan as the new director of the ICH Secretariat.

Pharmacy associates say more education regarding the transactional requirements of DSCSA is needed.

The agency clarifies its requirements for allowable excess volume and labeled vial fill size in injectables and biologics.

The European Pharmacopoeia adopts six new texts and revises 31 monographs in its 152nd session.

The International Conference on Harmonization finalizes Q&A document on APIs.

The agency streamlines risk and mitigation information.

The agency issues draft guidance on the development of drugs to treat Duchenne muscular dystrophy.

FDA recently issued a much-anticipated draft guidance on how to define and report established conditions in market applications.