
Regulatory flexibility can make continuous improvement possible.
Lois Atkins is a principal consultant, CMC regulatory affairs at Eli Lilly and Company

Regulatory flexibility can make continuous improvement possible.
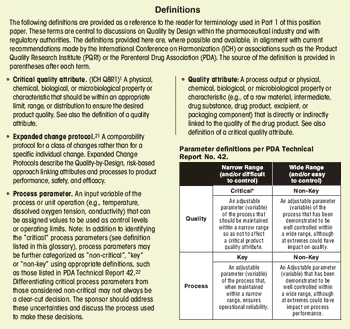
First in a three-part series that discusses the complexities of QbD implementation in biotech product development.

Published: January 1st 2010 | Updated:
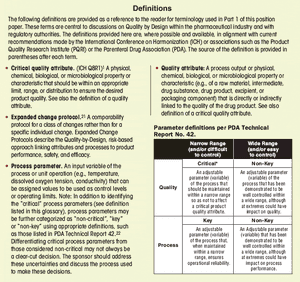
Published: November 1st 2009 | Updated: