
As automation in biomanufacturing becomes more important, so does the need to integrate process data.

As automation in biomanufacturing becomes more important, so does the need to integrate process data.

The USP Quality Institute’s third fellow in its Fellowship in Quality Medical Products program will study the role of poor-quality medicines in fostering antimicrobial resistance.

Getting to the root of the cause can prevent future problems, says Susan Schniepp, executive vice-president of post-approval pharma and distinguished fellow, Regulatory Compliance Associates.

To ensure the sterility of parenteral biopharmaceutical products, it is necessary to employ certain tools, technologies, and standard operating procedures.

The guidance describes how to develop a REMS Assessment Plan, discusses the impact of REMS on patient access to drugs, and gives recommendations on reporting REMS findings to FDA.

The investigation into the root cause of the NDMA impurity found in API is still ongoing, but the agency believes the impurities may be caused by specific chemicals and reaction conditions in manufacturing processes.

The plan details five goals to guide the development of Sentinel, a national electronic system for medical product safety surveillance established as part of the FDA Amendments Act of 2007, over the next five years.

The company is recalling 5 lots of Ceftriaxone for Injection, USP, 250mg; 10 lots of Ceftriaxone for Injection, USP, 500mg; 24 lots of Ceftriaxone for Injection, USP, 1g; and 3 lots of Ceftriaxone for Injection, USP 2g because of the presence of particulate matter in reconstituted vials.

FDA sent a warning letter to Skylark CMC Pvt. Ltd. after employees refused to let agency inspectors enter the company’s Ahmedabad, Gujarat, India facility.

Understanding the differences in inspection processes is the key to successful global expansion, according to Siegfried Schmitt, PhD, vice-president Technical, PAREXEL Consulting.

Asclemed USA Inc., dba Enovachem Pharmaceuticals is recalling the product due to labeling that incorrectly states that stoppers do not contain latex.

The agency sent a warning letter to Cao Medical Equipment Co., Ltd. after inspectors found CGMP violations at the company’s Langfang, Hebei facility.

FDA has withdrawn the proposed rule that would have allowed generic-drug makers to independently update and distribute new safety information in drug labels.

FDA cites Zhejiang Huahai Pharmaceutical in valsartan impurity investigation.

New FDA guidance developed to identify lapses in data integrity and promote best practices.
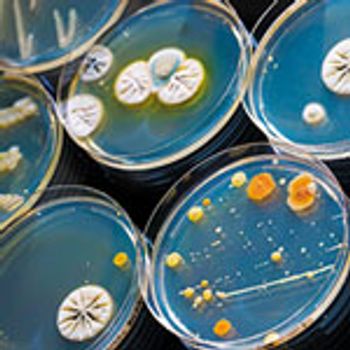
Microbial identity data can be critical for determining contamination sources.
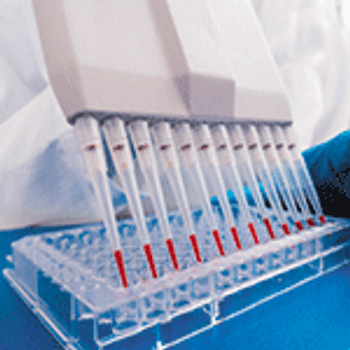
Unprocessed bulk material harvested directly from the bioreactor should be tested for contamination prior to downstream processing.

A required time frame should not be the driving force behind root cause investigations, says Susan Schniepp, executive vice-president of Post-Approval Pharma and Distinguished Fellow, Regulatory Compliance Associates.

Success depends on supplier communication and transparency, but it’s up to buyers to demand the right information and to look at the vendor’s overall business.

The partnership, co-funded by Enterprise Ireland, will develop technologies for monitoring the quality of biopharma processes.

The agency sent a warning letter to the company for marketing an unapproved stem cell product and CGMP violations.

The agency is developing a new way to assess, record, and report data from surveillance and preapproval inspections of sterile drugs.

The new program will provide cell and gene therapy companies a more efficient way to ensure quality compliance across collection center networks and to minimize quality system audit burden on these centers.

Building up relevant expertise in-house will make writing spec sheets for software easier, according to Siegfried Schmitt, principal consultant at PAREXEL.

Industry is searching for ways to deal with the criticality of ensuring data integrity.