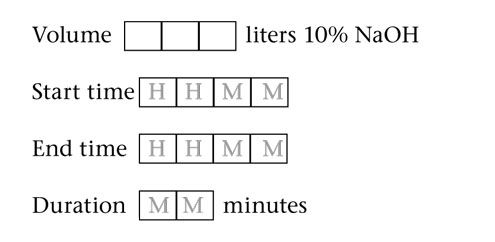
The National Institute of Standards and Technology (NIST) has developed Standard Reference Material (SRM) 2082 as a pathlength standard for UV absorbance measurements for use with the new generation of microvolume spectrophotometers and short-pathlength cuvettes.