
PharMEDium Services, LLC voluntarily recalled some lots of product because of a lack of sterility assurance.

PharMEDium Services, LLC voluntarily recalled some lots of product because of a lack of sterility assurance.

Conducting stability testing on APIs/finished drug product helps ensure shelf-life storage.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).

Enterprise-wide processes, procedures, and systems are the keys to data integrity and peace of mind, according to Siegfried Schmitt, PhD, principal consultant at PAREXEL.

The recall was initiated due to a product complaint in which white particulate matter, identified as mold, was discovered in a flexible bag from one batch.

FDA sent a warning letter to Deserving Health International Corp. after inspectors found CGMP violations including failure to prevent microbiological contamination.
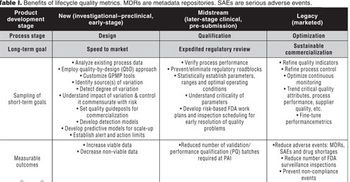
By improving data systems and quality metrics, businesses and company cultures can be strengthened.

An increasing number of warning letters shows that FDA is observing more problems with pharmaceutical contract manufacturing.

A survey by LNS Research and sponsored by Honeywell showed that industrial companies are not moving quickly to adopt cyber security; calls on CEOs to take action.

SOPs need to reflect a company’s specific manufacturing or other operations, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

A quality-by-design approach that implements PAT offers advantages in upstream cell-culture processing.

A roundtable Q&A with biopharma executives elucidates the challenges posed by single-use bioreactor bags in contributing to extractables and leachables in the biomanufacturing process.
Cross-functional reliability rooms identify risk and planning metrics, provide insights for production forecasts, and predict trends and areas for improvement.

The company is voluntarily recalling one lot of NEXTERONE 150 mg/100 mL Premixed Injection because of particulate matter found in the solution.

Investing in a single source enterprise-wide LMS to fuel risk management and mitigation offers multi-dimensional return on investment.

FDA is exploring ways to keep branded drug companies from using REMS to block the development of generic drugs.

The voluntary recall is due to blister packages containing the incorrect product.

Siegfried Schmitt, PhD, principal consultant, PAREXEL, discusses how to prepare for an inspection by a foreign regulatory agency.
Connectors are a critical element in the process optimization of single-use bioprocessing systems.

The level of formality in change control may be holding back your SOP progress, according to Siegfried Schmitt, principal consultant at PAREXEL.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.

FDA sent a warning letter to Ridge Properties, LLC, after inspectors found a variety of current good manufacturing practice violations at the company’s Salem, OR facility.

EDQM held a symposium on microbiology to gather industry feedback on alternative testing methods for microbiological control and sterilization processes.

The company is recalling several injectable products because of possible microbial contamination.
Detecting viral contaminants in biologic-based medicines-and identifying their source-requires a holistic testing approach.