
Cellular and gene therapy fields are currently on track for, if not already experiencing, a serious capacity crunch.
Ronald A. Rader is senior director, Technical Research, at BioPlan Associates.

Cellular and gene therapy fields are currently on track for, if not already experiencing, a serious capacity crunch.

FDA inspections can create uncertainty. Supervision of the contract manufacturer is crucial in ensuring compliance.
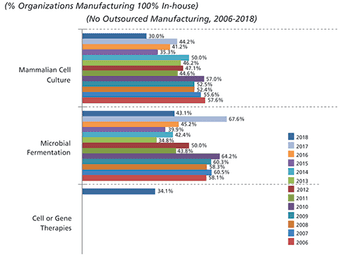
Outsourcing of manufacturing activities is expected to increase in 2019.

The growth in adoption of single-use systems for commercial manufacturing will be dramatic in coming years.

This article highlights 15 years of changes in biopharmaceutical manufacturing.
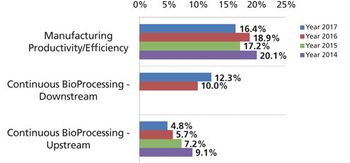
Development and adoption of new technologies create challenges that may take years to resolve.
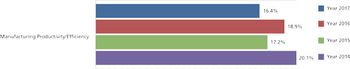
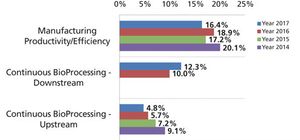
Innovation speeds discovery, drives down costs, and improves productivity.

Published: September 1st 2017 | Updated:
Published: March 1st 2018 | Updated:

Published: July 1st 2018 | Updated:
Published: November 1st 2018 | Updated:
Published: January 1st 2019 | Updated:

Published: May 1st 2019 | Updated: