
Bayer will make a $400-million upfront payment to develop and commercialize two anti-cancer compounds in Loxo Oncology’s portfolio.

Bayer will make a $400-million upfront payment to develop and commercialize two anti-cancer compounds in Loxo Oncology’s portfolio.

As part of the deal, J&J’s Janssen will pay an upfront payment of $50 million to research, develop, and commercialize up to six bispecific antibodies.

The company’s new modules offer scalable single-pass diafiltration and were exclusively showcased during its Leadership Forum series in Westborough, MA.

A new facility type integrates next-generation mobile cleanroom systems.
The use of therapeutic vaccines presents a new way to manage diseases, such as cancer and sexually transmitted diseases.

This article explores some of the challenges, services, and technologies that go into ensuring that vaccines are properly temperature controlled and maintain product integrity for delivery to patients.
Challenge trials may increase in coming years as new and improved challenge agents better emulate “natural” disease states.

Alternatives to time-consuming, error-prone operations promise to reduce vaccine manufacturing costs and improve facility flexibility.
Despite the challenges and high cost of development, vaccine innovation is at an all-time high, as new approaches aim to improve global access.

The use of approved platform technologies can reduce the time and cost required to generate new vaccines.

ADC Bio experts warn of impending problems in the ADC pipeline with millions wasted in development costs.

Bormioli Rocco Pharma offers new packaging solutions for powdered oral drugs, parenteral drugs, pediatric syrups, and protein-based drugs.

Company completes first successful run of what is believed to be the largest synthesis-scale done for oligonucleotide APIs.

Alcami will manufacture clinical supply of the API for a drug candidate targeting various hematologics and solid cancers.

The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.
This article discusses the potential of using high-productivity membrane chromatography to achieve intensified and flexible virus manufacturing.

The company expects to commercially launch the new vaccine in the US in the first quarter of 2018.

The company has officially opened a local brand office in South Korean to support its existing business in the region.

Supply Chain Wizard has developed a set of integrated digital solutions to improve supply chain functions.

The Human Vaccines Project has created the Universal Influenza Vaccine Initiative, a research program that will aim to understand the human immune system’s role in the development of universal influenza vaccines.

CDMO Celonic acquired a biomanufacturing facility in Heidelberg, Germany from Glycotope.

The companies will collaborate to develop and manufacture Grid’s lead therapeutic candidate for the treatment of solid tumors.

Reliable, high-quality products require innovative analytics and production.
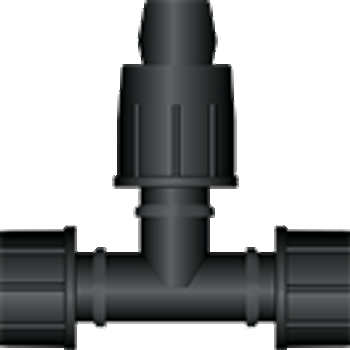
Connectors are a critical element in the process optimization of single-use bioprocessing systems.
Despite the disappointing therapeutic performance of ADCs thus far, the pipeline still boasts promising prospects.