
The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.

Amgen plans to invest approximately $300 million in a new biomanufacturing plant in the United States.

Shire will receive exclusive license to develop and commercialize AB Biosciences' pan receptor interacting molecule program for autoimmune and inflammatory diseases.

A collaboration between The Centre for Process Innovation and The Roslin Institute aims to develop commercially viable and scalable methods of producing biologics using transgenic animals.

The Flexicon PF7 peristaltic tabletop aseptic liquid-filling machine from Watson-Marlow Fluid Technology Group is suitable for GMP-regulated biotechnology and pharmaceutical operations.
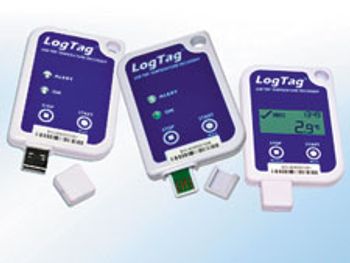
LogTag has added two new devices to its temperature data logger series.

Layout and supply details must be considered when implementing a fully disposable biopharmaceutical manufacturing process.
As closure integrity testing moves from a probabilistic to a deterministic basis, designs are promoting improved control and reduced operator contact.

Frustrated with high costs and drug shortages, hospitals adopt a DIY approach.

Greater clarity on the application of existing regulations will accelerate development of cell and gene therapies.
Single-use technologies are starting to gain ground as capacity needs change, but industrywide adoption remains low.

BioPharm International asked Andrew Bulpin, head of Process Solutions at MilliporeSigma, about the end-of-life options for disposable components of biopharmaceutical single-use systems.

The new Acquity Arc Bio System by Waters is specifically engineered to enable efficient transfer and improvement of bioseparation analytical methods.

A manufacturing disruption has led to an EpiPen shortage in Canada, which currently has no alternative auto-injectors available on the market.

The contract development and manufacturing organization has invested £30-million (US$42 million) at its Dundalk, Ireland site to offer expanded clinical trial services.

The company relocated its headquarters to Riverview, Florida.

Meissner Filtration Products has acquired PDC Aseptic Filling Systems, a supplier of aseptic filling systems and sealers, to expand Meissner's automated platform offerings.

Corning will feature its 3D bioprinting technology and tools to support 3D cell culture at booth #1029 during the SLAS 2018 conference on Feb. 3-7, 2018 in San Diego, CA.

Comecar will provide cell culture equipment for biopharmaceutical company CO.DON AG's new production site in Leipzig, Germany.

The company will invest $139 million in sterile manufacturing technology at its facility in Ballytivnan, Sligo, Ireland to support the growth of its oncology pipeline.

Clover Biopharmaceuticals will use GE's FlexFactory biomanufacturing platform with single-use bioreactors to produce innovative and biosimilar fusion protein products in China.

The funding from the Walloon Region will enable Univercells to start developing a manufacturing platform aimed at driving down costs of biosimilars manufacturing.

Lonza Pharma & Biotech's's Modular Automated Sampling Technology (MAST) platform, which allows for the collection of up to 10 sterile sample sources for automated analysis in multiple analytical devices, won the CPhI Pharma Award 2017 for Excellence in Pharma: Bioprocessing.

GE Healthcare will equip Cellular Biomedicine Group's cell therapy manufacturing facility in Shanghai, China, with its FlexFactory single-use platform, designed to speed up cell therapy manufacturing timelines.

Researchers at Purdue University and and industry experts have partnered up at the Advanced Lyophilization Technology Hub to optimize the 70-year-old freeze-drying process.