
Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.

Sartorius has delivered to ABL’s Strasbourg facility, a GMP viral vector manufacturing package solution that includes single-use bioreactors and an automation platform for normal flow filtration, tangential filtration, and mixing.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

Sensors and devices being developed by nGageIT Digital Health Solutions can track patient use of oral solid-dosage or injectable drugs.

The author reviews operative options for the implementation of a quality oversight and how companies can benefit from this function from a regulatory perspective.
A different perspective on controlling fixed costs of biomanufacturing, based on know-how from other industries, provides a competitive edge, says the CEO of Samsung BioLogics.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

Under this agreement, the companies will develop in parallel an antibody drug candidate and cell lines for other potential candidates.

GlobalData reports the need to shift away from egg-based manufacturing of vaccines in light of influenza-related deaths.

JLL, a real estate and investment management firm, recently released a new report on three trends shaping labs for the future of life sciences R&D.

Catalent completes $4.6 million expansion at Singapore clinical supply facility and marks 20 years in the region.
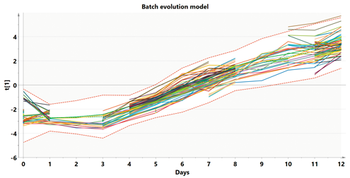
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

Lower costs, fewer opportunities for temperature excursions, and a smaller carbon footprint are making ocean transport more attractive for pharmaceuticals. Poseidon, a new collaborative pharma initiative, seeks to leverage benefits.

Alan Kennedy, executive director of TEAM UP, shared perspectives on Poseidon and ocean transport.

Spectroscopic tools present an alternative method for reliable at-line process monitoring and control.

Biologic new molecular entities (NMEs) accounted for 26% of total NME approvals in 2017.

The contract development and manufacturing organization has entered it first manufacturing contract worth $148 million for its recently completed Plant 3 facility.

The company is voluntarily recalling three lots of Labetalol Hydrochloride Injection, USP, 100 mg/20 mL Vial and one lot of Labetalol Hydrochloride Injection, USP, Novaplus because of the potential of cracked glass at the rim of the vials.

The company aims to add the additional analytical services in the European Union throughout 2018.

The new training center, the Jefferson Institute for Bioprocessing, will prepare engineering students and industry professionals for the field of biologics manufacturing.

Avid Bioservices will provide commercial manufacture of an enzyme replacement therapy by Roivant Sciences' Enzyvant subsidiary.

The agreement follows the recent opening of WuXi STA's oligonucleotide R&D labs in China and the US.

The companies have partnered to develop and commercialize vectorized antibodies against tau for Alzheimer's and other neurodegenerative diseases.

The study suggests that circumventing evolution in cell factories can enable the commercialization of new biobased chemicals to large-scale.

The company has expanded its single-use manufacturing capabilities at its facility in Fermoy, Ireland.