
Dujuan Lu, PhD, global leader—E&L, SGS Health Sciences, recommends some key issues that pharma manufacturers should consider when formulating products that require a medical device.

Dujuan Lu, PhD, global leader—E&L, SGS Health Sciences, recommends some key issues that pharma manufacturers should consider when formulating products that require a medical device.

According to USP, the products will advance quality and reduce risk in the development and manufacture of biologics and vaccines.
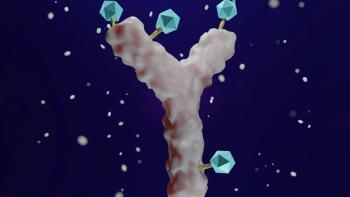
There is no such thing as a perfect linker.

Sara Fathollahi, PhD, product application specialist at DFE Pharma, makes the case of how excipient suppliers can support the transition from batch to continuous manufacturing.

Industry investments indicate increasing trust in the newest modalities, including mRNA approaches.

CPHI Barcelona has strong showing as bio/pharma industry moves confidently into 2024.

The final guidance document was issued to assist bio/pharma companies in the clinical development and licensure of COVID-19 vaccines.

Spark Therapeutics and SpliceBio have formed a collaboration to develop a gene therapy that can treat an inherited retinal disease.

The California Institute for Regenerative Medicine has partnered with Forge Biologics, which will manufacture AAVs to help accelerate gene therapy programs in California.

Wheeler plans to use the facility to bolster its mission in helping fledgling biotech innovators and startups accelerate their development at an equitable price.

Chris Spivey, editorial director, hosts a wide ranging discussion on the COVID-19 pandemic response, future government communications, and healthcare funding for mRNA with experts Dr. Kate Broderick, Chief Innovation Officer, Maravai LifeSciences and Dr. Tom Madden, President & CEO at Acuitas Therapeutics. Highlights include supply chain modalities, ensuring a fully trained manufacturing workforce, combining delivery with gene editing innovations, epigenetic approaches to modulate gene expression moving toward the clinic. We conclude with the huge potential for RNA expression of monoclonal antibodies and protein replacement applications.

Sander Van Gessel, MEng, Business Unit Director, DFE Pharma, discusses the important components of nitrosamine impurities from a manufacturing point of view as a part of his session, "Reducing Nitrosamines Without the Use of Scavengers: The Critical Role of Excipients - An Excipient Manufacturer's View."

Sander Van Gessel, MEng, Business Unit Director, DFE Pharma, discusses the recent findings of nitrosamine impurities in human drugs as a part of his session, "Reducing Nitrosamines Without the Use of Scavengers: The Critical Role of Excipients - An Excipient Manufacturer's View."

Under an expanded agreement, Cellares will provide proof-of-concept manufacturing for a second CAR-T cell therapy from Bristol Myers Squibb.

Salipro Biotech and Icosagen have entered into a multi-target collaboration to discover and characterize monoclonal antibodies.

Under this agreement, Acuitas Therapeutics’ LNP technology platform will be transferred to BIOVECTRA for use in manufacturing mRNA-based therapies.

Roche will gain exclusive worldwide rights to develop, manufacture, and commercialize Ionis’ investigational RNA-based therapeutic candidates for Alzheimer's and Huntington's disease.

The program will improve access to adeno-associated virus gene therapy vectors.

New routes to cancer treatment can be found with the help of tumor-infiltrating lymphocyte (TIL) therapies.

Orakl Oncology has raised funds to develop its precision oncology platform and accelerate drug development.

Eli Lilly and Company has received a complete response letter from FDA for its anti-dermatitis biologic therapeutic, lebrikizumab.

The program will allow sponsors of certain CBER and/or CDER-regulated products more frequent communication with FDA staff.

The prize was awarded jointly to Katalin Karikó and Drew Weissman for their groundbreaking discovery regarding modification of the bases in mRNA.

Pfizer has entered into a collaboration with Ginkgo Bioworks to discover novel RNA molecules across priority research areas.

Government provides a spoonful of sugar, and genuine leadership, for good medicines.