
Two general chapters on elemental impurities? limits and procedures are to become official Feb. 1, 2013, with implementation proposed for May 1, 2014.

Two general chapters on elemental impurities? limits and procedures are to become official Feb. 1, 2013, with implementation proposed for May 1, 2014.

Neil Lewis, chief technology officer at Malvern Instruments, talks about the challenges associated with biologics.

A recent ISPE guidance provides a baseline for the design of quality laboratory facilities.

An introduction to a new series on manufacturing within global markets.
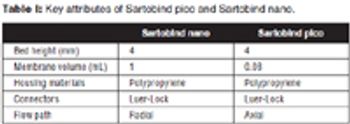
The authors describe the development of an ultra scale-down anion exchange membrane adsorber, and demonstrate scalability to larger-scale devices.

This month, Sharon Strause, an industry consultant, provides a look back at "Computer System Validation Part I: Testing and Verification of Applications Software" by Leonard J. Goren.

USP revises labeling requirements for Heparin.
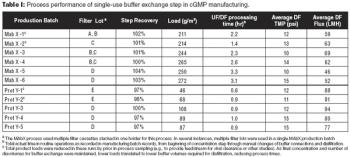
This case study describes the process used to transition from a multi-use system to single-use tangential flow filtration for performing final buffer exchange steps.
Insights on single-use systems implementation and exploitation in biopharmaceutical manufacturing and processing, based on a QbD approach.

NIBRT's Jayne Telford provides an overview of biopharmaceutical analytics and their accompanying qualification and validation steps.

Patrick Jackson of Vindon Scientific offers key considerations for choosing an outsourced sample storage facility.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.
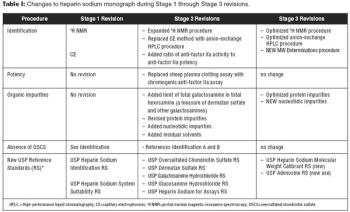
USP optimizes identification tests and impurities procedures.

USP Hosts Symposium on Science and Standards

Using a competency-based approach to effectively train biopharmaceutical industry staff.

The authors demonstrate how an integrated model is helping to achieve regulatory flexibility. This article is part of a special section on biopharmaceutical trends.

Brazil's regulatory health authority, Anvisa, plans to establish quality requirements for locally produced pharmaceutical excipients, Anvisa told BioPharm International.

A one-day sign off for batch records is considered a best practice in the industry.

Recently developed immunoassay technology platforms reduce sample volume requirements and improve cycle times.
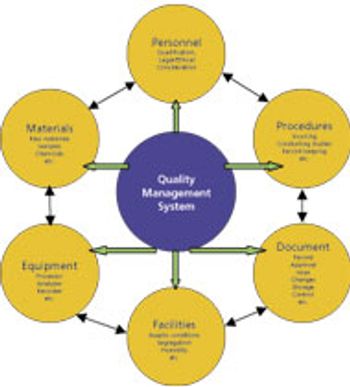
Members from an ASQ working group provide analytical methods to enable PAT.

Unnecessary analytical testing can lead to unnecessary costs.
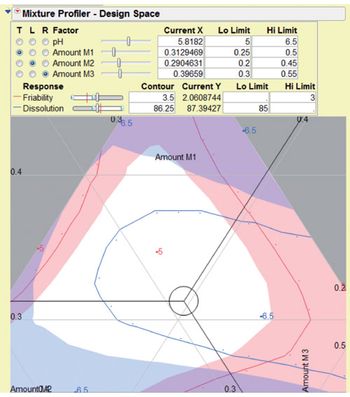
Harmonized regulations call for a risk-based and systematic approach to evaluating and selecting CPPs.

Leading industry collaborators outline top 10 best practices for human error reduction.

In a special anniversary interview, Washington Editor Jill Wechsler speaks with with FDA Deputy Commissioner Deborah Autor about where the agency is headed.

The contract provider needs to know as much as the NDA holder.