
Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

Programs assist in the fast track of drugs for serious conditions.

ISPE study reveals quality systems of manufacturing as the leading cause of drug shortages.

EMA?s revised guideline on biosimilars containing biotechnology-derived proteins is published for public consultation.

USP appoints regulatory experts to elemental impurities implementation advisory group.

FDA cites cGMP violations of finished pharmaceuticals at the company's facilities in Marion North Carolina and Jayuya, Puerto Rico.

FDA Discovers Microbial Contamination in Compound Pharmacy Products

The company is cited for using unapproved visual-inspection methods for finished parenteral drugs and conducting inadequate visual inspections.

A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.

FDA addresses shortages of drugs needed to treat premature infants and patients unable to eat or drink by mouth.

Company receives notice from FDA for not fully investigating foreign particles in APIs and finished products from its facility in Ingelheim am Rhein, Germany.

USP defers implementation date to work closely with ICH Q3D. USP will also form a new advisory group for implementation of the new general chapters on elemental impurities.

The U.S. Pharmacopeial Convention is offering free online access to public standards to help ensure the quality of the herbal ingredients used in medicinal products.

FDA has released guidance on best practices for conducting and reporting pharmacoepidemiologic safety studies.

New Center for Pharmaceutical Advancement and Training increases number of experts and available tools in Sub-Saharan countries.

Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.

KR Karu from Sparta Systems spoke with BioPharm International about the importance of having an enterprise quality management system.

FDA issues draft guidance to minimize medication errors.

FDA inspections of compounding pharmacies manufacturing sterile-drug products lead to voluntary recalls.

FDA's FY 2014 budget includes more than $10 million above the 2012 budget for inspections of products and ingredients manufactured in China.

Prefilled-syringe line features automation and novel disinfection techniques.
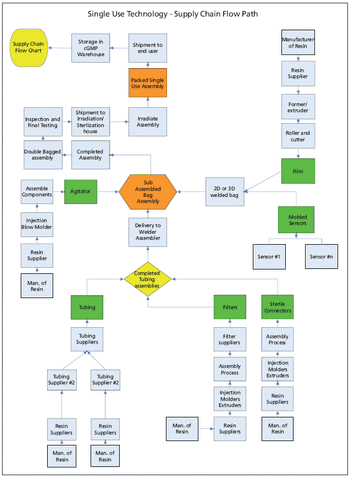
The authors suggest techniques for mitigating risk and securing the supply chain for single-use components used in biopharmaceutical manufacturing.

EU authorities are stepping up their efforts to incorporate QbD principles.

The authors describe the growth characteristics of human mesenchymal stem cells cultured in a stirred-tank bioreactor.

The authors present solutions based on a review of current service offerings and their audit experience.