
The agency sent a warning letter to A-S Medication Solutions LLC after it found the company didn’t comply with drug listing file requirements.

The agency sent a warning letter to A-S Medication Solutions LLC after it found the company didn’t comply with drug listing file requirements.

FDA Commissioner Scott Gottlieb announced the July meeting as part of his commitment to address opioid abuse.

FDA sent a warning letter to drug compounder DCA, Inc. dba Beacon Prescriptions for failing to ensure sanitary conditions.

The three regulatory agencies have agreed to data requirements for development of new antibiotics.

In its June meeting, the agency’s Pharmacovigilance Risk Assessment Committee discussed medicine safety reviews.

FDA asked Endo Pharmaceuticals to remove Opana ER from the market, citing the potential for abuse.

Yusef Manufacturing Laboratories, LLC received an FDA warning letter citing CGMP violations at its Clearfield, UT facility.

The agency published an action plan to nurture innovation and drug development by SMEs.

The agency released guidance for industry regarding the United Kingdom’s withdrawal from the European Union.

This article reviews systems and processes that enable a laboratory to approach troubleshooting in an effective way, while also taking a proactive, preventive approach to managing atypical laboratory scenarios.

Approval of breakthrough therapies requires expedited quality assessment.

Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

As more companies decouple drug substance from finished drug manufacturing operations, an integrated approach can ensure safe, reliable logistics for frozen storage and shipping.

Increased understanding of potential impurities has spurred efforts to standardize monitoring procedures.

How statistical methods and novel indices can be used to monitor and benchmark variability, to guide continuous improvement programs.
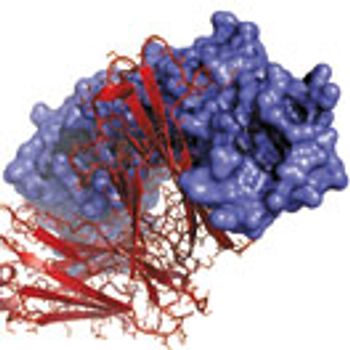
An increase in biologics raises awareness of particle generation and its role in negative patient outcomes.

The company was cited for cGMP violations at its Irvine, California facility.

The agency met with the representatives of the East African Community to discuss the creation of a networking agency.

CBER is moving forward with the development and approval of regenerative medicine advanced therapies.

FDA’s new commissioner asks staff for suggestions for fighting the opioid crisis.

The agency has given a green light to a new system for collecting and monitoring suspected adverse reactions.

The agency’s Committee for Medicinal Products for Human Use recommended AstraZeneca’s brodalumab for the treatment of moderate-to-severe plaque psoriasis.

BioPharm International speaks with a CMO to find out how it is coping with capacity challenges, regulatory authority scrutiny, supply chain reliability, and new requests from clients to incorporate continuous manufacturing into processing lines.

Effective communication between contract manufacturing organizations and pharmaceutical company clients relies on well-defined master service and quality agreements.

FDA’s focus on the quality culture and its request for quality metrics may ensure a successful company-CMO relationship.