
The European Medicines Agency has granted Samsung BioLogics approval to manufacture a monoclonal antibody at the company’s second facility in Songdo, Incheon, South Korea.

The European Medicines Agency has granted Samsung BioLogics approval to manufacture a monoclonal antibody at the company’s second facility in Songdo, Incheon, South Korea.

The agency has approved Nucala (mepolizumab) to treat Eosinophilic Granulomatosis with Polyangiitis (EGPA) to GlaxoSmithKline. This indication is the first FDA-approved therapy specifically to treat EGPA.

FDA has accepted for review Eli Lilly and Company’s biologic drug candidate that is in development for treating migraine.
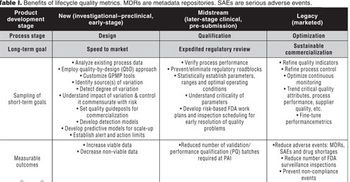
By improving data systems and quality metrics, businesses and company cultures can be strengthened.

An increasing number of warning letters shows that FDA is observing more problems with pharmaceutical contract manufacturing.

A survey by LNS Research and sponsored by Honeywell showed that industrial companies are not moving quickly to adopt cyber security; calls on CEOs to take action.

The agency and the European Commission published updated guidance to answer questions about Brexit.

During the November meeting of the agency’s Pharmacovigilance Risk Assessment Committee, review of Esmya (ulipristal acetate) was announced as well as warnings about prostate cancer treatments.

Biopharma majors are among the industry stakeholders who have commented and raised questions about FDA’s recently proposed draft guidance for analytical assessment of similarity in biosimilars.

FDA has approved Mylan’s biosimilar to Roche’s blockbuster anti-cancer biologic, Herceptin.

In a recently released statement, FDA Commissioner Scott Gottlieb warned of an amino acids supply shortage and gave an update on a saline supply shortage, both due to the impact of hurricanes on Puerto Rican manufacturing facilities.

New gene therapies and combination products require innovative regulatory approaches.

SOPs need to reflect a company’s specific manufacturing or other operations, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Biopharma employees reveal employment objectives, opportunities, and frustrations.

A quality-by-design approach that implements PAT offers advantages in upstream cell-culture processing.

A roundtable Q&A with biopharma executives elucidates the challenges posed by single-use bioreactor bags in contributing to extractables and leachables in the biomanufacturing process.
Cross-functional reliability rooms identify risk and planning metrics, provide insights for production forecasts, and predict trends and areas for improvement.

Experts slam drug prices and endorse government price negotiations and curbs on drug advertising.

Manufacturers and trading partners struggle to meet drug tracking requirements

The new guidelines discuss GMPs and patient protection specific to advanced therapy medicinal products.

Following a vote by member states in favor of Amsterdam as the agency’s new headquarters, the relocation will take place over the next 16 months, with operations expected to start up in March 2019.

The agency has approved a biologic-based new molecular entity for treating hemophilia A and has expanded the indication for a leukemia drug to now treat a common form of non-Hodgkin lymphoma.

Commissioner Scott Gottlieb, MD, outlined a plan to support innovation of regenerative medicines while ensuring public safety.

The company is voluntarily recalling one lot of NEXTERONE 150 mg/100 mL Premixed Injection because of particulate matter found in the solution.

Investing in a single source enterprise-wide LMS to fuel risk management and mitigation offers multi-dimensional return on investment.