
While most pharma companies are in partial production using generators, few are operating at 100% capacity.

While most pharma companies are in partial production using generators, few are operating at 100% capacity.

The voluntary recall is due to blister packages containing the incorrect product.

The agency gave Breakthrough Therapy Designation to GSK’s GSK2857916 monotherapy for the treatment of patients with multiple myeloma who have failed at least three prior lines of therapy.

FDA sent warning letters to four companies illegally selling products online that claim to prevent, diagnose, treat, or cure cancer without evidence to support these statements.

Reliable, high-quality products require innovative analytics and production.

Siegfried Schmitt, PhD, principal consultant, PAREXEL, discusses how to prepare for an inspection by a foreign regulatory agency.
Connectors are a critical element in the process optimization of single-use bioprocessing systems.

FDA seeks to focus on problematic facilities and inform firms quickly about site problems.

The level of formality in change control may be holding back your SOP progress, according to Siegfried Schmitt, principal consultant at PAREXEL.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.

A mutual recognition agreement to accept inspection data from eight EU regulatory authorities may reduce inspection burdens at global drug facilities.

The vaccine is approved for the prevention of shingles in adult patients aged 50 years and older.

FDA sent a warning letter to Ridge Properties, LLC, after inspectors found a variety of current good manufacturing practice violations at the company’s Salem, OR facility.

Scott Gottlieb, MD, went before the House Committee on Energy and Commerce to give members the agency’s view on how to fight the opioid crisis, stressing the use of long-term treatment with drug therapy.

The M&A advisory firm has developed an interactive map of manufacturing sites to give insight into the market size of global manufacturing.

The agency has published educational material for physicians promoting the benefits of biosimilars.
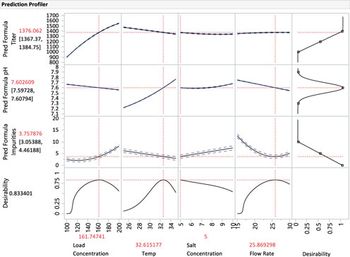
This article examines how process characterization is crucial to process understanding and then applying that understanding to controlling a process.

EDQM held a symposium on microbiology to gather industry feedback on alternative testing methods for microbiological control and sterilization processes.

The company is recalling several injectable products because of possible microbial contamination.

The European Commission and EMA have created an action plan to address challenges identified by stakeholders involved in developing advanced therapies.

This approval marks the second gene therapy to be approved by FDA and the first to be approved for certain types of non-Hodgkin lymphoma.

The agency has published a continuity plan for its move from the United Kingdom.

The vaccine is a non-live, recombinant subunit vaccine that combines an antigen and an adjuvant system to trigger a targeted and long-lasting immune response to the shingle-causing virus.

Stelara (ustekinumab), already approved for treating adults, is now also approved for treating adolescents with moderate to severe plaque psoriasis.

Through the support of the Japanese agency, Daiichi Sankyo intends to further develop its genetic vaccine platform focused around its new nucleic acid delivery technology.