
The agency recommended a total of 11 drugs at its July meeting, including five orphan drugs.

The agency recommended a total of 11 drugs at its July meeting, including five orphan drugs.

The agency cited the Italian company for aseptic processing failures.

The National Academies of Sciences, Engineering, and Medicine released a report on its findings on the opioid abuse epidemic, citing the need for a prescribing culture change.

BIO and PhRMA issued statements expressing the groups’ approval of the continuation of user fee programs.

The agency sent a warning letter to Vista Pharmaceuticals Limited for CGMP failures related to equipment cleaning and process control.

One day after Ocular Therapeutix sent FDA a detailed response highlighting manufacturing improvements, FDA rejected its application for Dextenza, a new drug designed to alleviate post-operative eye pain.

In an FDAVoice blog post, the CDER director highlighted two recent scientific advances.

The agency’s Pharmacovigilance Risk Assessment Committee made recommendations on the risk of multiple sclerosis treatments, injectable methylprednisolone medicines, and the use of gadolinium contrast agents.

FDA sent a warning letter to Shandong Analysis and Test Center for CGMP violations including data integrity issues and insufficient testing procedures.
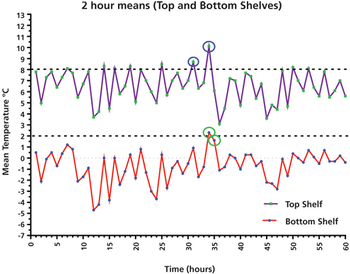
This column presents a data case study of a laboratory refrigerator and its qualification performance over five days, with important lessons for using average and individual results, as well as user requirements.

The opening presentation gives the company a chance to put their best foot forward, according to Siegfried Schmitt, principal at PAREXEL.

This article presents a general strategy for authorship of deviation investigations, with primary focus on regulatory inspection success.
While the measurement of the toxicity of leachables is not always a required parameter, the information collected during these studies could inform future bioprocessing runs.

Single-use bags containing toxic or hazardous materials required special handling.

CDER’s Janet Woodcock endorses modern drug manufacturing to ensure access to safe and reliable medicines.

Continuous processing of 100 g of monoclonal antibody in 24 hours has been demonstrated using lab-scale equipment.

Electronic systems can remove opportunities for individuals to make mistakes or to manipulate the data.

Optimize practices and meet requirements using electronic data integrity systems.
Short tandem repeat profiling is the most validated method to confirm cell line identity and avoid misidentified or cross-contaminated cell lines.

The agency announced a plan to eliminate its existing orphan designation request backlog.

The directorate highlights its 2016 achievements.

The agency announced it is taking steps to increase competition within the prescription drug market.

Fagron Sterile Services has voluntarily recalled three lots of Succinylcholine Chloride 20mg/mL 5mL syringe to the hospital/clinic level.

The agency recommended approval of treatments for hepatitis C, cancer, multiple sclerosis, and arthritis.

FDA Commissioner Scott Gottlieb announced the agency will hold a public meeting on drug competition as part of its Drug Competition Action Plan.