
As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

As regulators strive for balance in cGMPs for cell, gene, and tissue therapies, risk-management principles must guide decisions involving process media and additives.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the value of internal audits and how the information gained can be applied.

Industry fears limited benefits as FDA readies voluntary data tracking program.

The complex nature of biologics adds additional CQAs that must be determined to ensure the safe development of biologics
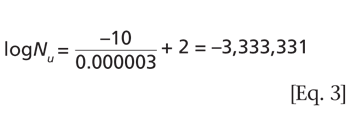
Understanding the purpose of the biological indicator can guide the development of an effective sterilization process.

The mAb is the first approved treatment that targets the progressive form of the disease.

The agency sent a warning letter to Opto-Pharm Pte Ltd. after deficiencies in sterile manufacturing procedures were found at the company’s Singapore facility.

A new report states that wider access to new antivirals for HCV and better screening of patients would help avert 90,000 deaths by 2030.

Manufacturers and regulators are working to reach consensus on the harmonization of management of postapproval changes.

The agency recommended six drugs for approval in March 2017 including treatments for neuroblastoma, heart failure, and more.

The agency is recommending the suspension of a variety of medications because of unreliable bioequivalence studies conducted by Micro Therapeutic Research Labs

The taskforce will be evaluating how data can be used to support pharmaceutical research, innovation, and development.

Will new generic drugs bring the cost of medicines down in the way policy makers hope?

In a FDAVoice blog post, CBER Director Peter Marks discusses the new designation for cell therapies that treat life-threatening diseases.

FDA issued a warning letter to USV Private Limited citing CGMP violations that include data integrity and microbiological contamination issues.

The two pharmacopeias signed a Memorandum of Understanding as recognition of their collaboration for developing international science-based standards.

The agency sent a warning letter to Badrivishal Chemicals & Pharmaceuticals detailing several CGMP violations.

Trump’s choice for FDA commissioner faces drug pricing, regulatory, and approval challenges.

The European Medicines Agency met with European and African regulators to discuss how to improve the availability of safe and effective drugs beyond Europe.

The agency granted Pfizer, Cellectis, and Servier clearance to begin clinical development with UCART19.

The agency cited Morton Grove Pharmaceuticals for inadequate quality control procedures.

The agency sent a warning letter to Chongqing Pharma Research Institute Co., Ltd. citing data integrity violations.

Dr. Janet Woodcock said implementation of Informatics Process Management is a priority during the latest Director’s Corner podcast.

The Mutual Recognition Agreement will allow FDA and EU inspectors to recognize each other’s work and avoid the duplication of drug inspections.

President Trump calls for faster FDA approvals and lower drug prices.