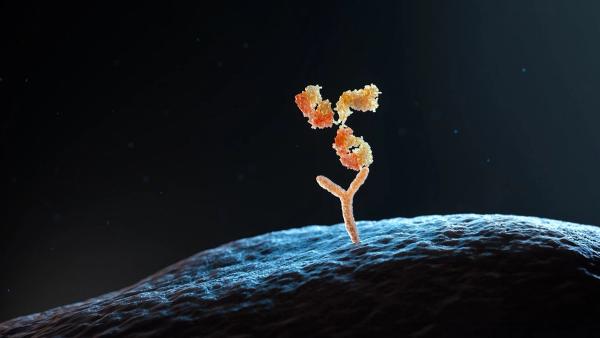
Manufacturing
Latest News
Latest Videos
More News

Webinar Date/Time: Mon, Apr 7, 2025 2:00 PM EDT

Edwin Stone, PhD, CEO of Cellular Origins, offers his unique perspective to the challenges facing CGT manufacturers, drawing on his background in robotics and life science automation.

Advances in automation technologies and bioprocessing equipment are driving fuller adoption of automation in biomanufacturing.
This study evaluates the best-in-class elastomer compounds, formulations, and coatings, affixed with aluminum seals and press-on caps to Type I borosilicate tubular glass vials conforming to the International Organization for Standardization (ISO 8362) injection containers requirements.

The benefits include reduced contamination risks, simplified validation processes, and streamlined operations.

The investment, led by Foresight Group and secured alongside funding from PARP, will be used to further commercialize uFraction8’s microfiltration technology.

Takeda’s TAKHZYRO (lanadelumab) is now approved in Europe as a subcutaneous injection treatment for hereditary angioedema in patients 12 years old and above and in adults.

RNA modalities will especially benefit from fuller adoption of continuous manufacturing platforms.
Being fully versed on the ins and outs of design quality can improve the project design phase life cycle.

Artificial intelligence, among other technological advances, is pushing innovation boundaries.

BioPharm International® chatted with Preeya Beczek, managing director and co-founder of Beczek.COM, about industry trends from 2024 and which of those might impact the industry in 2025, including the big trend of AI.

Previous domestic capital expansion commitments since 2020 had been made in Research Triangle Park and Concord, both in North Carolina; Kenosha County, Wisconsin; Lebanon, Indiana; and Lilly’s home city of Indianapolis.

Single-use solutions are the company’s focus this year, including tubing and custom-molded parts.
Jason C. Foster, CEO and executive director of Ori Biotech, spoke on addressing bottlenecks in CGT manufacturing and what to expect in the CGT manufacturing space moving forward.

Jason C. Foster, CEO and executive director of Ori Biotech, discusses the milestone achievement in commercial-scale CGT manufacturing with the company’s IRO platform.

BioPharm International® sat down with Kate Coleman from Arriello to run through the major trends from 2024, including the importance of technological advances, and how these may progress in 2025.

Webinar Date/Time: Tue, Mar 4, 2025 11:00 AM EST

William Oh, MD, led a panel discussion during which the practicalities of incorporating AI tools into the daily workflow of busy clinicians were discussed.

BioPharm International® sat down with Adam Sherlock, CEO of Qinecsa, to discuss the changing political landscapes in the US and Europe and how that may affect the bio/pharma industry.

BioPharm International® spoke with Adam Sherlock, CEO of Qinecsa, about the industry trends of 2024, the future technology agenda, and M&A prospects in 2025.

Personalized medicine is a trend that continues to impact innovation and business decisions in the biopharma industry.

Development of new modalities of biotherapeutic molecules will rely on manufacturing, regulatory, and collaborative support.
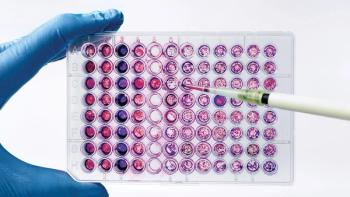
Cell-line engineering and media formulation improvements are leading to greater performance.
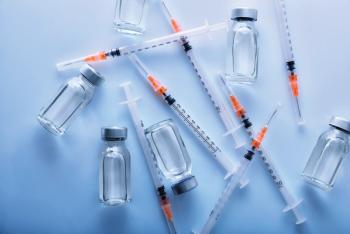
Rapid market growth in biologics is helping the packaging market expand as well, but the complex nature of biologics and the desire for personalized therapies present unique hurdles to make packaging solutions cost-effective and safe for patients.

Enduro Genetics will use the funding to expand its cell programming technology for scalable biomanufacturing with microbial cells at its Copenhagen, Denmark site.