Manufacturing
Latest News
Latest Videos

More News

Maximizing value in advanced therapies requires the use of proven approaches, expert logistics, and tailored distribution, says Miguel Forte, board member of ARM.

ARM Board Member Miguel Forte highlights CGT investment shifts, sector resilience, and alternative funding strategies during Meeting on the Mesa.

Cellevate’s nanofiber technology enables higher viral vaccine titers in adherent cell cultures, advancing efficiency in large-scale biomanufacturing.

Each step of development and manufacturing is becoming more interconnected, and BioPharm's September/October issue highlights the strategies, tools, and innovations shaping the future of the field.

Webinar Date/Time: Wed, Nov 5, 2025 11:00 AM EST

This second part of a two-part article provides a clear understanding of microbiological load reduction during cleaning processes in the non-sterile pharmaceutical manufacturing.
Jon Ellis, CEO, shares his thoughts on Trenchant BioSystems’ new technology, its reception within the cell and gene therapy sector, and the future nature of industry partnerships.

Comparing our survey results with recent headlines uncovered biopharma’s digital transformation paradox: high potential vs. low adoption.

In a session at the Cell and Gene Meeting on the Mesa, Prime Medicine CEO Allan Reine discussed how prime editing offers versatile, safe gene correction, but that delivery to target cells remains a major hurdle.
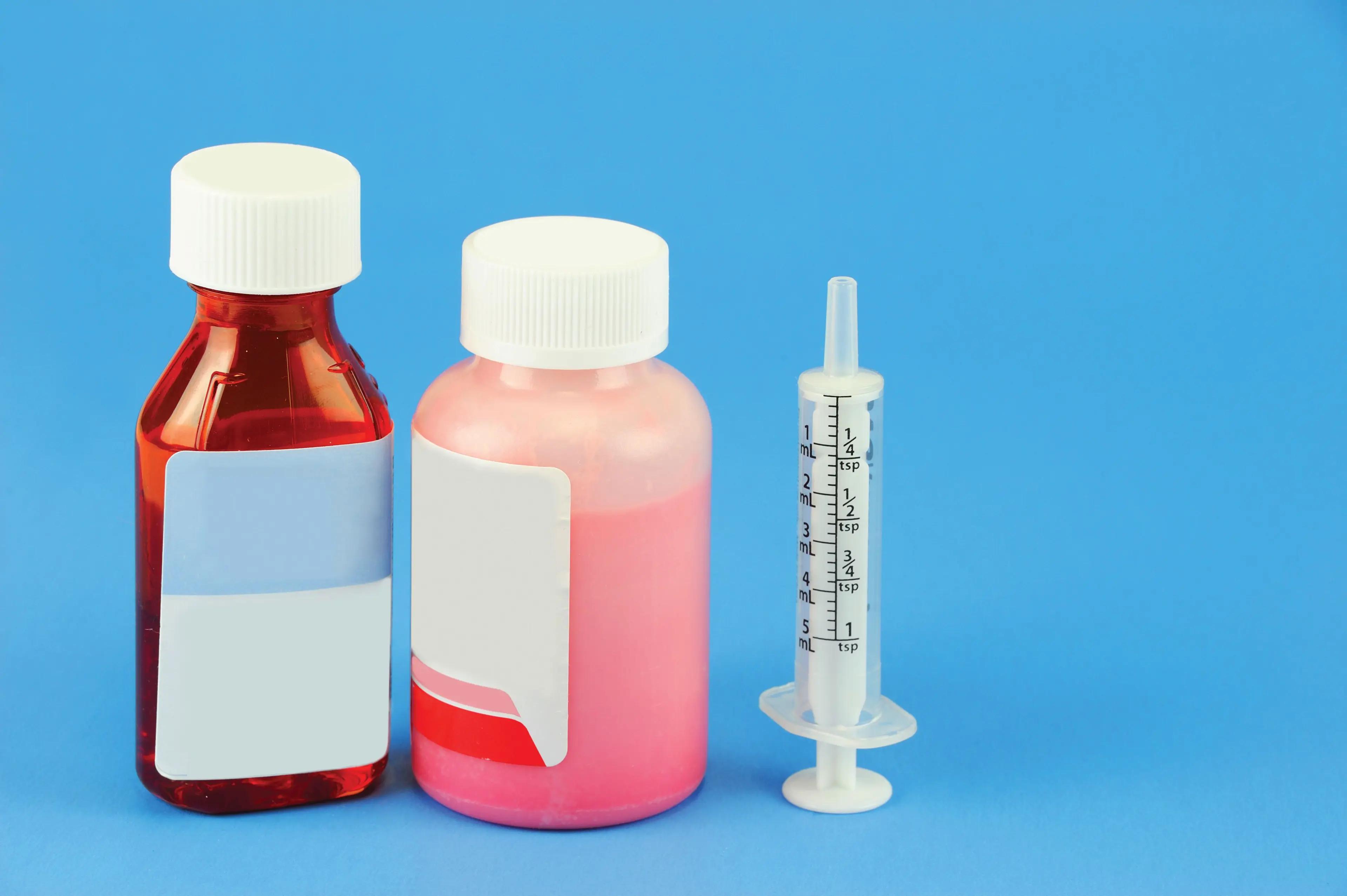
A growing demand for liquid medicines is driven by patient groups' unique needs, improving compliance through flexible and palatable dosing options.

A panel at the Cell and Gene Meeting on the Mesa discussed how advanced therapy production demands modular platforms, automation, and data governance to drastically improve patient access and affordability.

Naobios and Olon France create custom HCP ELISA to advance vaccine trials and strengthen global biopharmaceutical manufacturing capacity.

On July 31, President Donald Trump said the federal government would “deploy every tool in our arsenal to protect American families from continued abusive drug pricing practices” should companies fail to comply.

Webinar Date/Time: Wed, Oct 22, 2025 11:00 AM EDT

Novartis expands its immunology pipeline with the Tourmaline acquisition and tests direct-to-patient model to reshape drug manufacturing strategies.

The 100% tariff on imported drugs will pressure biopharma companies to build manufacturing sites in the US or face significant costs.

The new protein-based HDR enhancer aims to improve CRISPR precision for advancing cell and gene therapy development workflows.
RION partners with Lonza for CGMP manufacturing of PEP, advancing exosome drug development and scalable biopharma production.

The company’s mission to be “continuously innovative” has resulted in the creation of this novel acronym.
Alvin Jogasuria, ProBio; Matthew Lunning, University of Nebraska Medical Center; and Carl Schoellhammer, DeciBio, go behind the headlines to discuss the need for doing more with less.

New GS knockout CHO-K1 systems accelerate biologics manufacturing by improving yields, scalability, and flexibility for biopharma development programs.
ACIP votes to separate MMR and varicella vaccines (MMRV) for children to cut febrile seizure rates.

Engineered biosynthetic pathways provide new avenues for accessing complex molecules.

A collaboration between Limula and Institut Paoli-Calmettes aims to advance automated stem cell transplant processing to improve cryoprotectant removal, enhance patient outcomes, and streamline manufacturing.

Optimizing FDA 483 responses with strategic CAPA creates resilient quality compliance in biopharma manufacturing.