Manufacturing
Latest News
Latest Videos

More News

Modular and replication strategies are being utilized to meet growing demand for CDMO facilities.
Fluorescence spectroscopy offers unique advantages for characterizing EVs.

With FDA's clearance, Kedrion can manufacture Ryplazim (plasminogen, human-tvmh), the first and only FDA-approved therapy for treating PLGD-1, at its Bolognana, Italy, site.

The company officially launched new production suites, a revamp of its development labs, and more at its Thousand Oaks, Calif., cell therapy manufacturing facility.

With this $3.6 million investment, the CDMO will strengthen its advanced labeling, automated visual inspection, and fill/finish technology.

Webinar Date/Time: Thu, Dec 5, 2024 11:00 AM EST

The investment will allow Sanofi to strengthen antibody bioproduction at its Lyon Gerland site in France.

Webinar Date/Time: Thursday, December 5th, 2024 at 11:00 AM EST

Under the collaboration, the companies will create and test circVec DNA–LNP formulations with an eye toward potential therapeutic applications.

The new European facilities located in German, Italy, and Lithuania will specialize in drug discovery and R&D services.

Under a £15.7 million (US$20.7 million) investment, SEKISUI has expanded its UK site for clinical-grade drug substance manufacturing.

With financing led by OrbiMed, Novo Holdings, and Jeito Capital, Alentis Therapeutics will develop a pipeline of Claudin-1-targeted ADCs to treat solid tumors.

Empowered by a $1.5 billion commitment from a major investor, Frontier Scientific Solutions has unveiled its plans for establishing a global free trade zone gateway intended for facilitating the life sciences supply chain using Wilmington International Airport in North Carolina.

Avantor has launched its new, expanded Innovation Center in Bridgewater, NJ, with integrated workflows under one roof and purpose-built collaboration spaces.

Avantor executives discuss the future of the biopharmaceutical industry and the impact that a wave of next-generation biotherapeutics will bring.

Ginkgo Bioworks has achieved the first milestone in an ongoing partnership with Merck, known as MSD outside of the United States and Canada, aimed at improving biologics production.

TrakCel's OCELLOS, an IT platform, has been selected to orchestrate the administration of five out of seven autologous or matched allogenic cell therapy products approved or expected to be approved in 2024.

With the launch of the Institute for Cell Therapy Discovery and Innovation, the MD Anderson Cancer Center will bring together expertise in developing cell therapies for cancer, autoimmune diseases, and infections.

Lonza’s Synnafix has licensed its ADC technology to BigHat Biosciences, which will combine it with its ML design platform to generate newly designed ADCs.

The Novo Nordisk Foundation has committed DKK 600 million (US$87.4 million) towards the initial costs of the center housing the new supercomputer, which has the potential to accelerate drug discovery innovation.

The collaboration aims to develop precision genetic medicines using ViaNautis’ proprietary polyNaut technology platform.

The company is investing $76 million to increase ADC manufacturing capacity at its St. Louis, Mo. facility.
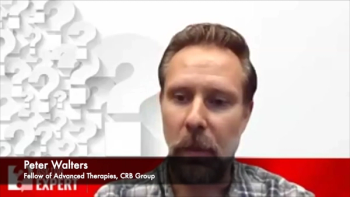
In this episode of the Ask the Expert video series, Peter Walters, Fellow of Advanced Therapies at CRB Group, discusses how facilities used for solid dosage manufacturing may be retrofitted into sustainable cell and gene therapy production facilities.

BioPharm International spoke with Kenneth LaRiviere, head of Engineering at Andelyn Biosciences, about the possible problems that can arise when operating an older facility for biopharmaceutical manufacturing.
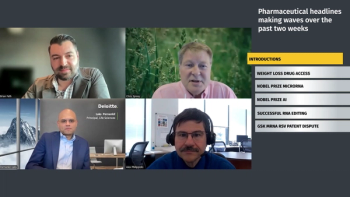
Laks Pernenkil, Brian Feth, and Alex Philippidis go behind the headlines to discuss the impact of recent news, including FDA’s drug shortage list update, Nobel prize winners in microRNA and AI, and a big-potential research win for RNA editing.