
Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

Grace's ProVance disposable Protein A chromatography column is designed for downstream purification.

With SMEs gaining wider recognition as the powerhouse of research and innovation in Europe, regulatory agencies are urging companies to engage with regulators early in the drug-development process.
Antibody fragments pose unique challenges in recovery, purification, and formulation.

Data from BioPlan Associates' 10th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production suggest that the interest in disposable devices has begun to extend to biopharma operations beyond basic single-use bags and connectors.

BioReliance opens Clearance Services Laboratories in Maryland for downstream bioprocessing studies.

What the industry's future holds and what needs to be done to get there.
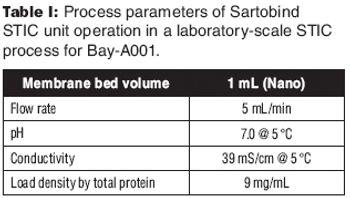
A new downstream purification platform using a salt-tolerant membrane adsorber.

NIBRT's Ian Nelligan on what to expect when starting a downstream process.

The author reviews the state of downstream processing, including a look at the streamlining of full processes and borrowed technologies.
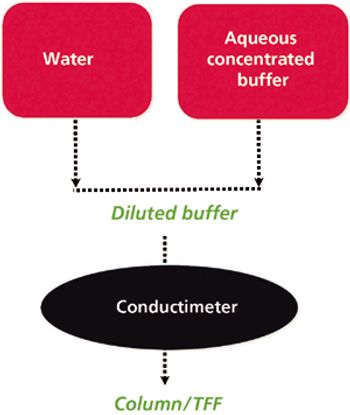
The author describes recent developments to help overcome the downstream-processing bottleneck.
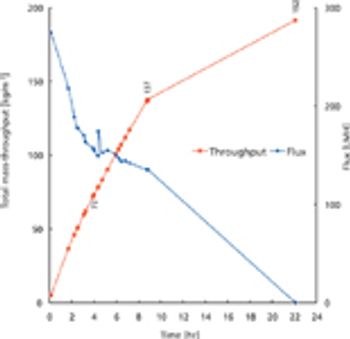
Newer classes of biotherapies will require innovations in processing technology.

A high-performance anion exchange resin performs well compared with membranes. In addition, the resin offers greater flexibility and cost savings.

This summary provides an introduction to our special issue on Separation and Purification.

Careful analysis of an unusual precipitate identifies defects.

Can vendor-user partnerships overcome the typical wait-and-see attitude toward new technologies?
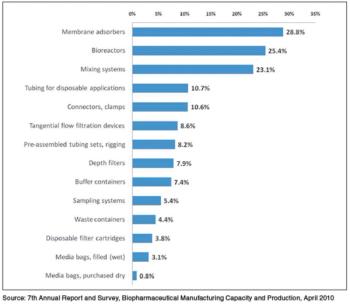
Although single-use systems are widely used in upstream unit operations, their acceptance in downstream processes has been slow.
An analysis of flow rate, load density, viral clearance, and cost.
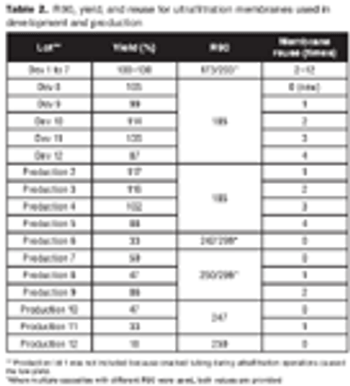
How to balance porosity, plugging, and lot-to-lot variability in filters.
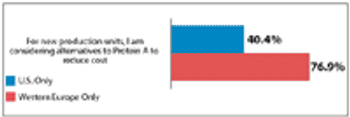
Biomanufacturers and vendors are now exploring more cost-effective purification technologies.
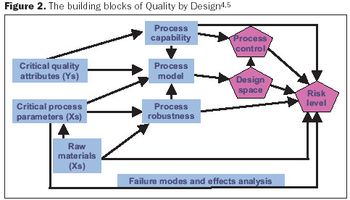
To speed up the downstream process, you must get the right data, in the right amount, at the right time. Here's how.
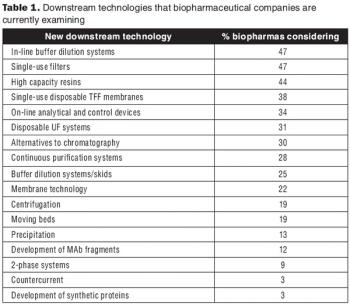
The new technologies being developed to improve downstream systems go beyond traditional chromatography.
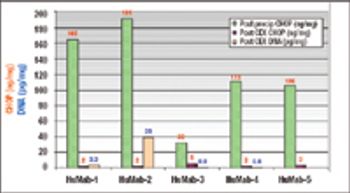
Precipitation prior to capture chromatography offers a simple, robust, and economical method to remove CHO host cell proteins and DNA.
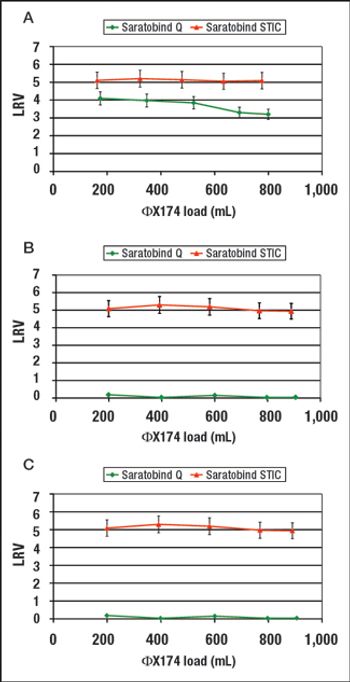
STIC allows polishing to be carried out without an interstitial dilution step, which reduces process time and avoids additional buffer preparation and hold steps.

Ten biopharmaceuticals gained marketing authorization in the US or EU in 2008, although only four were new approvals.
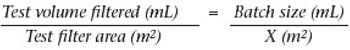
Why staining is crucial in flow decay studies.