
Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

Laboratory test methods evaluate cleaning agents and cleaning process design for removing resin residues from the surfaces of non-dedicated chromatography columns systems.

The latest advances in downstream processing include remote monitoring, membrane chromatography technology, single-use sensors, and new uses of data analytics.

Downstream process equipment for mAbs manufacturing must be designed to fit technology developments in upstream processes.

Parker Bioscience Filtration added a cleanroom at its site in Birtley, UK for manufacturing single-use assemblies and sensors used in biopharmaceutical processing.
New ligands are being developed to meet the separation and purification needs of next-gen biologics.

A key technology that can help achieve a continuous production flow is single-pass tangential flow filtration.

The investment builds on a collaboration the companies entered into in 2007 for various biomanufacturing projects.

GE’s new facility, which will be operational in 2019, will produce a fiber-based chromatography platform for more efficient biopharmaceutical purification.
The second half of this article describes the testing methods used by the authors to demonstrate the applicability of single-use mixing technology for virus inactivation.

The companies partnered to build a 500-L single-use pilot-scale plant for biologics production.

The maturation of single-use technologies presents commercial bioprocessing options for small-volume drug products.
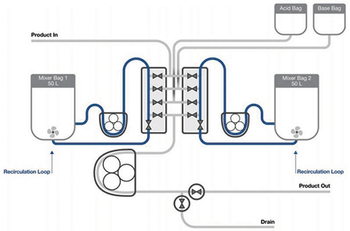
Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

Automation can improve many aspects of bioprocessing, but several hurdles must be overcome before the full range of benefits can be realized.

The media, by Tosoh Bioscience, is composed of calcium and phosphate buffers and offers mixed-mode properties for biomolecule purification.

The acquisition of Flex Concepts adds custom, single-use products to Entegris’ single-use bag product line.

Increasing demand for biologics is driving the need for innovation in bioprocessing.

The new resin used a combination of “jetting” technology and a high-performance Protein A ligand.

The company’s next-generation ultraperformance liquid chromatography platform is designed to meet the evolving laboratory requirements for chromatographic performance.

Silicone is one of the polymeric materials that can be used for single-use components and assemblies in biopharmaceutical manufacturing.

Higher cell titers and cell densities have posed a challenge to cell harvesting, a crucial step in biologics manufacturing.

Parker Bioscience will expand laboratory, cleanroom, office, and warehouse facilities at its Birtley, UK manufacturing site.