
A statement from FDA Commissioner Scott Gottlieb and Deputy Commissioner Anna Abram detailed FDA’s new plan to advance plant and animal biotechnology innovation.

A statement from FDA Commissioner Scott Gottlieb and Deputy Commissioner Anna Abram detailed FDA’s new plan to advance plant and animal biotechnology innovation.

Kimberly-Clark Professional added the Kimtech A5 Sterile Integrated Hood and Mask XL for head shapes, sizes, and hairstyles that pose a challenge to standard aseptic gowning for cleanroom operators.

The acquisition will allow Lonza to further develop technology for scalable autologous cell-therapy manufacturing.

Bioconjugation requires aseptic manufacturing and containment for cytotoxic payloads.

The company will collaborate with GlycoBac to offer an insect cell line for the development of viral vaccines and gene therapies.

The new facility will include comprehensive mammalian process development and manufacturing capabilities.

The companies will develop and commercialize ARO-HBV, a Phase I/II subcutaneous, ribonucleic acid interference therapy candidate being investigated for the treatment of chronic hepatitis B viral infection.

The companies signed a three-year clinical manufacturing agreement to manufacture GlaxoSmithKline’s specific peptide enhanced affinity receptor T-cell receptor therapy for United States, Canadian, and European clinical trials.

The UCL-Pall Biotech Centre of Excellence will address industry challenges and provide workforce training.

The companies will work together to discover, develop, and commercialize immunotherapies for patients with solid-tumor cancers in a collaboration worth $695 million per program.

The companies partnered to build a 500-L single-use pilot-scale plant for biologics production.

The maturation of single-use technologies presents commercial bioprocessing options for small-volume drug products.
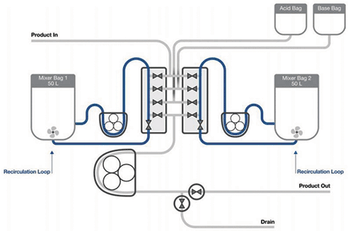
This article explores the use of single-use mixing technology in a detergent-based virus inactivation step during a monoclonal antibody production process.
Non-destructive surface area measurement can improve stability testing.

Specialist biopharmaceutical company, Alvotech, has announced receipt of a manufacturing license from the Icelandic Medicines Agency, applying to its biopharmaceutical facility based in Reykjavik, Iceland.

A collaboration using Pall’s bioprocessing technology in G-CON’s PODs enables flexible continuous bioprocessing and viral vector facility solutions.
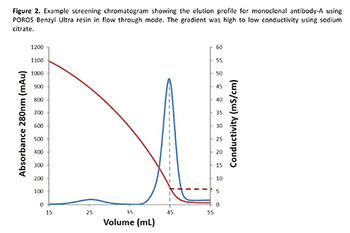
Hydrophobic interaction chromatography (HIC) in flow-through mode offers a more efficient and cost-effective polishing/purification process to remove monoclonal antibody aggregates while maintaining purity at ≥99% than a mixed-mode bind/elute procedure.

While food, shelter, and clothing are the primal essentials for life, hope-as embodied by modern medicine-has now become part of that human expectation.
Testing demonstrates an automated semi-continuous process strategy for viral inactivation with steps that mimic batch processing.

GE Healthcare, Cobra Biologics, and the Centre for Process Innovation (CPI) have entered into a collaboration to advance manufacturing of adeno-associated virus vectors for gene therapy.

The sterile-manufacturing contract development manufacturing organization is approved by FDA for viral vector manufacturing fill/finish processing at its biologics facility in Scotland, UK.

FDA awarded nearly $3 million in grants for continuous manufacturing and other advanced manufacturing technologies as part of the agency’s efforts to ensure a robust and reliable supply of biological products.

The CHF 400-million (US$416-million) investment in Lonza's biopark in Visp, Switzerland, will expand Ibex Solutions with two new offerings, drug substance development and drug substance and drug product manufacturing.

BeiGene is set to build late-stage clinical and commercial production capacity for cancer monoclonal antibodies with GE Healthcare’s KUBio, the prefabricated biopharma facility based on single-use technologies.

The biopharmaceutical company will invest approximately $800 million to expand facilities and manufacturing capacity at its campus in Rensselaer County, NY.