
Continuous downstream bioprocessing is proving its worth, but connecting different operations and integrating upstream remains a challenge.

Continuous downstream bioprocessing is proving its worth, but connecting different operations and integrating upstream remains a challenge.
For cellular materials, new ultra scale-down devices inform large-scale downstream processing techniques.

Chromatography modeling can enhance bioprocessing efficiencies.

The decision to use disposable bioreactors is now driven by commercial rather than technological considerations.
Although Protein A remains a top technology for monoclonal antibody purification, the industry continues to look for new approaches to improve conventional capture chromatography.

The membrane-based Protein A purification tool was unveiled at the 2017 PepTalk Conference in San Diego, California.
In addition to having the optimal cell line and process, it is crucial to have the optimal cell culture medium and feed to maximize performance potential.
Advances in technology are increasing the productivity and efficiency of commercial-scale chromatography bioprocesses.

Industry experts discuss best practices for selecting a separation technology.

MilliporeSigma and the International Vaccine Institute in Seoul, South Korea aim to develop more robust, scalable vaccine manufacturing processes.
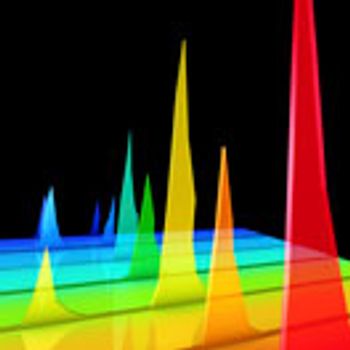
The authors provide application data to support the use of SEC beyond small-scale operations.

Industry experts provide insights on the challenges and importance of using buffers in downstream processing.

Novasep is building a new synthesis laboratory and adding capacity for kilogram-scale batches of synthetic molecules that are needed for biological testing and preclinical trials, at its Pennsylvania, US facility.

The authors explore the use of precipitation using polyvinyl sulfonic acid and zinc chloride in place of capture chromatography to reduce the cost of goods in the insulin manufacturing process.

BioPharm International sat down with Kevin Isett, PhD, co-founder and CEO of Avitide, to find out why he thinks the company’s tailored approach to purification resins will change the face of biopharmaceutical separation.

A new consortium involving Arecor, FUJIFILM Diosynth Biotechnologies and the Center for Process Innovation will focus on formulation innovation as a way to improve downstream processing and reduce biopharmaceutical cost.

The UK’s National Biologics Manufacturing Centre will use Novasep’s BioSC Lab for protein purification.

This case study reviews how quality-by-design principles can be implemented in an intermediate chromatography purification step that uses cation-exchange chromatography.Abstract

Industry experts discuss the development of process chromatography in bioprocessing.
New single-use technologies and other filtration systems are beginning to address cost, throughput, and manufacturing footprint demands.

The company’s purification platform is thought to reduce the number of purification steps that are currently required for the manufacture of complex therapeutics.

Optimization of each phase in a chromatographic cycle has a positive impact on productivity.

The new sizes follow the 2014 launch of the company’s fully disposable purifier.

The new column features Natrix’s signature macroporous hydrogel.

BioSC Lab biochromatography system performs protein purification in batch and continuous modes.