
Flush solutions for liquid chromatography mass spectrometry (LC-MS) systems and ultrapure solvents for ultra-high-performance LC-MS systems from Thermo Fisher Scientific can minimize interference and maximize lab instrument uptime.

Flush solutions for liquid chromatography mass spectrometry (LC-MS) systems and ultrapure solvents for ultra-high-performance LC-MS systems from Thermo Fisher Scientific can minimize interference and maximize lab instrument uptime.

Shimadzu’s Imagereveal MS mass spectrometry imaging data analysis software can analyze large sets of data or simultaneously analyze multiple sets of data.

The TSQ Fortis Triple Quadrupole Mass Spectrometer from Thermo Fisher Scientific offers fast, robust liquid chromatography-mass spectrometry (LC-MS/MS) analysis for clinical research laboratories.

More complex biologic samples must be evaluated to ever higher levels of specificity and sensitivity.

Materials in contact with a drug must be fully characterized to ensure they do not negatively affect the safety and efficacy of the product.

Sciex’s latest additions to its biopharma portfolio helps to optimize workflow for better analytics in the lab.

The company’s new LCMS-9030 system is designed for high resolution and accurate mass detection.

The company unveiled its latest innovations to its mass spectrometry portfolio at ASMS in San Diego.

Process analytical technology tools have enabled manufacturers to monitor and control their production processes.

This article discusses affinity capture for tier determination and surveys a broad collection of commonly and less commonly used assays to analyze cell culture media
The National Institute of Standards and Technology (NIST) has developed Standard Reference Material (SRM) 2082 as a pathlength standard for UV absorbance measurements for use with the new generation of microvolume spectrophotometers and short-pathlength cuvettes.

Access to multiple analytical techniques is essential for fully characterizing complex protein formulations.

Experts share insights on the various methods used for purity and impurity analysis of therapeutic proteins.

The critical quality attributes of biotherapeutics must be monitored to ensure product safety and efficacy.
The authors present a robust and easy-to-implement chromatography column performance assessment method, called direct transition analysis (DTA).
This article summarizes the approaches, challenges, and future perspectives for the characterization of N-glycans in biopharmaceutical products.

Despite limitations, mass spec is having an impact on biologic drug development and manufacturing.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.
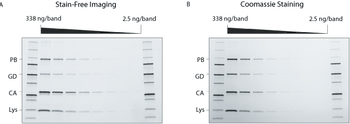
Innovations in electrophoresis and chromatography upstream of protein characterization can accelerate research.

A step-wise process is used to characterize glycans and understand the functioning of a molecule for biosimilar development.

Time and sensitivity are essential for analytical technologies in all phases of biopharma development.
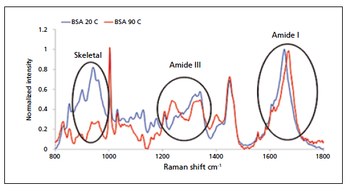
In this article, the author reviews some of the techniques that can yield valuable information on protein stability, focusing specifically on protein aggregation. Emphasis is placed on the enhanced information made available when technologies are used orthogonally, and the alignment of different approaches with specific stages of the biopharmaceutical development workflow.
The authors present a review of the techniques commonly used for glycosylation analysis.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.