
As biopharma enjoys success, it cannot ignore pressing patient access questions.

As biopharma enjoys success, it cannot ignore pressing patient access questions.

Jim Miller, President of PharmSource, speaks with BioPharm International.

Eric Langer, Managing partner at BioPlan Associates, speaks with BioPharm International about emerging trends in cell therapy.

If adopted, open innovation, beyond outsourcing and licensing, could boost biopharm pipelines four-fold, a recent Deloitte study suggests.

EvaluatePharma report shows continued pharma and biotech sector confidence.

Important IP contractual provisions should be included when working with CMOs.

As the biopharma industry awaits FDA’s guidance on biosimilar naming, brand and generic manufacturers establish positions.

The method patent covers the infusion of bendamustine hydrochloride for the treatment of certain blood cancers.

Supply chain consortium establishes a working group to address quality problems in India.

The Australian pharmaceutical market offers opportunities for manufacturers despite challenges.

jayk7/Getty Images

Mallinckrodt Pharmaceuticals has bought Ikaria, a privately-held critical care company, for $2.3 billion.

Changes in the country’s political landscape may affect the pharmaceutical industry market in the future.
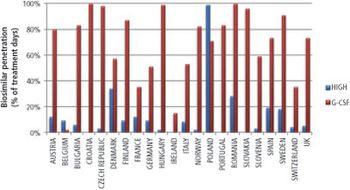
Investors are lining up for the biosimilars market as patents reach expiration and regulatory pathways are defined.

Manufacturers are under pressure to develop pipelines, promote quality, and justify pricing.

Although competing therapies will continue to be released in the immune-oncology space, efficacy profiles, combination regimens, and administration setting may influence a drug’s preferred status more than price.

Pharmacy benefit manager Express Scripts offers Viekira Pak at a discount and excludes the drug’s higher-priced competitors from its formulary.

On December 8, Pfizer announced that it will establish a research program in gene therapy, and collaborate with Spark Therapeutics in Philadelphia, to develop potential gene therapy treatments for hemophilia.

Enhanced R&D efforts and the growing manufacture of finished-dosage drugs in India will shape the country's future success, according to a new report from CPhI.

Japanese drugmaker Otsuka announced plans to buy CNS-specialist Avanir Pharmaceuticals for $3.5 billion in an all-cash tender offer.

Biopharma employees report gains in compensation and job satisfaction, as industry growth continues.

Biotech drug manufacturers' emails may have been hacked by individuals with sophisticated investment banking skills, according to a report from the New York Times.

Merck announced an agreement with NewLink Genetics to acquire exclusive rights to its experimental Ebola vaccine, rVSV-EBOV, for $50 million.

Despite recent changes in corporate tax law, Ireland remains a valuable and attractive partner in the development and manufacture of biopharmaceuticals.

Approximately 40% of total global growth will come from specialty medications, according to a new report from the IMS Institute for Healthcare Informatics.