
The company’s board of directors has appointed Aron Knickerbocker as the new president and CEO to succeed Lewis Williams, MD, PhD, effective Jan. 1, 2018.

The company’s board of directors has appointed Aron Knickerbocker as the new president and CEO to succeed Lewis Williams, MD, PhD, effective Jan. 1, 2018.

Russian biotechnology company BIOCAD plans to enter the European market with oncological and autoimmune medicines.

Girish Malhorta, CPhI expert and president of EPCOT International, discussed world revenues for pharmaceuticals and how companies may improve cost and availability of drugs in CPhI’s 2017 Annual Report.

The new center will serve as a regional hub where scientists and engineers can work with customers for biological development.

The company is considering options for a full or partial separation of its Consumer Healthcare business, either through a spin-off, sale, or other transaction.

Reeling from financial and tropical storms, Puerto Rico needs stable industry to aid its recovery.

Cell-based therapies are moving medical treatments forward, but intellectual property uncertainties may delay progress.

Faced with divisive political and social issues, Congress must find a way to reach consensus.

New reports address biopharma’s leading concerns: funding for drug development and pricing of finished drugs.

CDER’s Janet Woodcock endorses modern drug manufacturing to ensure access to safe and reliable medicines.

How has the bio/pharmaceutical contract manufacturing industry evolved and changed over the years and what does the future hold?

BIO report measures decade-long investment and acquisition trends for emerging biotech companies.

Drug sales forecasts fall for first time in 10 years, thanks to pressures to reduce drug prices and the advent of biosimilars.

New reports indicate that drug prices are slowing compared to other healthcare costs.

The 2017 Global Healthcare Leaders Survey suggests that value-based and risk-sharing payment could have an even more transformative impact on the industry than scientific breakthroughs.

In Lazard's Global Healthcare Leaders 2017 survey, most respondents said pricing and reimbursement were the most significant challenge, but nearly half of the pharma executives who responded see value-based pricing as having the greatest impact on the business, potentially even more than scientific breakthroughs.

Biomedical researcher shares insights from a career dedicated to advancing therapeutic innovations for unmet medical needs.

Pharmaceutical companies need to radically change to survive and thrive amid shifting healthcare and technology changes.
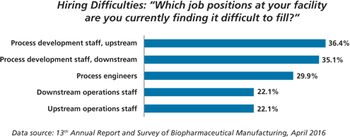
New study shows China biopharma companies face staffing shortages.

Philadelphia plays host to the exhibits, conferences, and networking activities of CPhI North America on May 16-18, 2017.

An influx of millennial workers may have an impact on whether pharma manufacturers choose to implement IIoT technology.
A controversial naming convention attempts to explain important distinctions between biologic drugs and their biosimilar counterparts.

Pharmaceutical Technology spoke with Tommy Fanning, head of biopharmaceuticals for IDA Ireland, to get a perspective on how Brexit may affect the pharmaceutical industry.

Reducing regulatory roadblocks requires more than the stroke of a pen.

Report: Global medicine spending will reach nearly $1.5 trillion by 2021.